Gas-Phase Ozone Oxidation of Monoterpenes: Gaseous and ...
Gas-Phase Ozone Oxidation of Monoterpenes: Gaseous and ...
Gas-Phase Ozone Oxidation of Monoterpenes: Gaseous and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
246 JIANZHEN YU ET AL.<br />
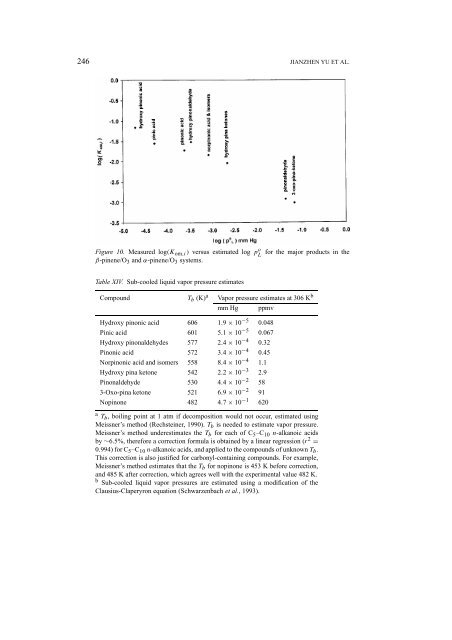
Figure 10. Measured log(K om,i ) versus estimated log pL o<br />
β-pinene/O 3 <strong>and</strong> α-pinene/O 3 systems.<br />
for the major products in the<br />
Table XIV. Sub-cooled liquid vapor pressure estimates<br />
Compound T b (K) a Vapor pressure estimates at 306 K b<br />
mm Hg<br />
ppmv<br />
Hydroxy pinonic acid 606 1.9 × 10 −5 0.048<br />
Pinic acid 601 5.1 × 10 −5 0.067<br />
Hydroxy pinonaldehydes 577 2.4 × 10 −4 0.32<br />
Pinonic acid 572 3.4 × 10 −4 0.45<br />
Norpinonic acid <strong>and</strong> isomers 558 8.4 × 10 −4 1.1<br />
Hydroxy pina ketone 542 2.2 × 10 −3 2.9<br />
Pinonaldehyde 530 4.4 × 10 −2 58<br />
3-Oxo-pina ketone 521 6.9 × 10 −2 91<br />
Nopinone 482 4.7 × 10 −1 620<br />
a T b , boiling point at 1 atm if decomposition would not occur, estimated using<br />
Meissner’s method (Rechsteiner, 1990). T b is needed to estimate vapor pressure.<br />
Meissner’s method underestimates the T b for each <strong>of</strong> C 5 –C 10 n-alkanoic acids<br />
by ∼6.5%, therefore a correction formula is obtained by a linear regression (r 2 =<br />
0.994) for C 5 –C 10 n-alkanoic acids, <strong>and</strong> applied to the compounds <strong>of</strong> unknown T b .<br />
This correction is also justified for carbonyl-containing compounds. For example,<br />
Meissner’s method estimates that the T b for nopinone is 453 K before correction,<br />
<strong>and</strong> 485 K after correction, which agrees well with the experimental value 482 K.<br />
b Sub-cooled liquid vapor pressures are estimated using a modification <strong>of</strong> the<br />
Clausius-Claperyron equation (Schwarzenbach et al., 1993).