Gas-Phase Ozone Oxidation of Monoterpenes: Gaseous and ...
Gas-Phase Ozone Oxidation of Monoterpenes: Gaseous and ...
Gas-Phase Ozone Oxidation of Monoterpenes: Gaseous and ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
252 JIANZHEN YU ET AL.<br />
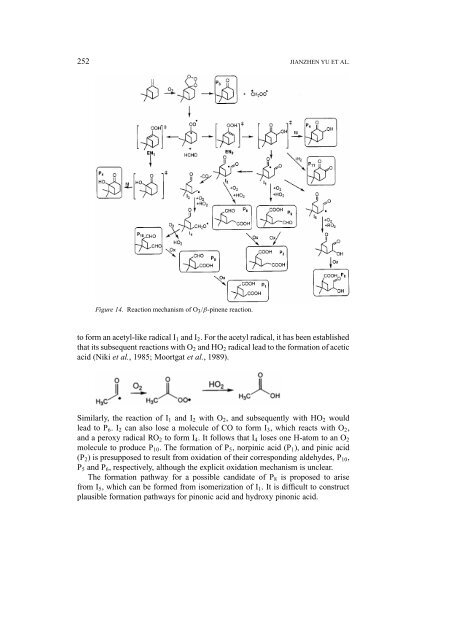
Figure 14. Reaction mechanism <strong>of</strong> O 3 /β-pinene reaction.<br />
to form an acetyl-like radical I 1 <strong>and</strong> I 2 . For the acetyl radical, it has been established<br />
that its subsequent reactions with O 2 <strong>and</strong> HO 2 radical lead to the formation <strong>of</strong> acetic<br />
acid (Niki et al., 1985; Moortgat et al., 1989).<br />
Similarly, the reaction <strong>of</strong> I 1 <strong>and</strong> I 2 with O 2 , <strong>and</strong> subsequently with HO 2 would<br />
lead to P 6 .I 2 can also lose a molecule <strong>of</strong> CO to form I 3 , which reacts with O 2 ,<br />
<strong>and</strong> a peroxy radical RO 2 to form I 4 . It follows that I 4 loses one H-atom to an O 2<br />
molecule to produce P 10 . The formation <strong>of</strong> P 5 , norpinic acid (P 1 ), <strong>and</strong> pinic acid<br />
(P 2 ) is presupposed to result from oxidation <strong>of</strong> their corresponding aldehydes, P 10 ,<br />
P 5 <strong>and</strong> P 6 , respectively, although the explicit oxidation mechanism is unclear.<br />
The formation pathway for a possible c<strong>and</strong>idate <strong>of</strong> P 8 is proposed to arise<br />
from I 5 , which can be formed from isomerization <strong>of</strong> I 1 . It is difficult to construct<br />
plausible formation pathways for pinonic acid <strong>and</strong> hydroxy pinonic acid.