Gas-Phase Ozone Oxidation of Monoterpenes: Gaseous and ...
Gas-Phase Ozone Oxidation of Monoterpenes: Gaseous and ...
Gas-Phase Ozone Oxidation of Monoterpenes: Gaseous and ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
254 JIANZHEN YU ET AL.<br />
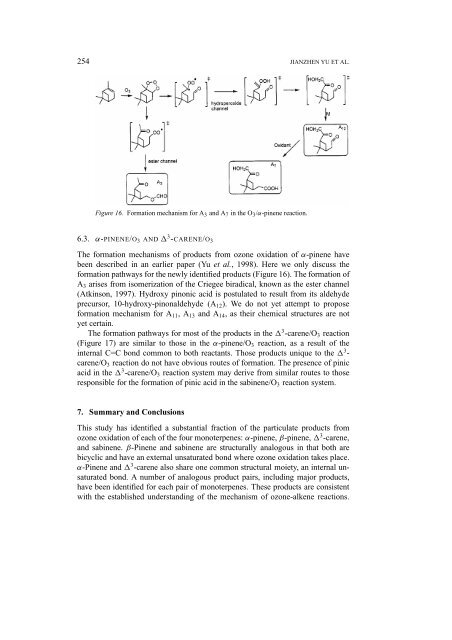
Figure 16. Formation mechanism for A 3 <strong>and</strong> A 7 in the O 3 /α-pinene reaction.<br />
6.3. α-PINENE/O 3 AND 3 -CARENE/O 3<br />
The formation mechanisms <strong>of</strong> products from ozone oxidation <strong>of</strong> α-pinene have<br />
been described in an earlier paper (Yu et al., 1998). Here we only discuss the<br />
formation pathways for the newly identified products (Figure 16). The formation <strong>of</strong><br />
A 3 arises from isomerization <strong>of</strong> the Criegee biradical, known as the ester channel<br />
(Atkinson, 1997). Hydroxy pinonic acid is postulated to result from its aldehyde<br />
precursor, 10-hydroxy-pinonaldehyde (A 12 ). We do not yet attempt to propose<br />
formation mechanism for A 11 ,A 13 <strong>and</strong> A 14 , as their chemical structures are not<br />
yet certain.<br />
The formation pathways for most <strong>of</strong> the products in the 3 -carene/O 3 reaction<br />
(Figure 17) are similar to those in the α-pinene/O 3 reaction, as a result <strong>of</strong> the<br />
internal C=C bond common to both reactants. Those products unique to the 3 -<br />
carene/O 3 reaction do not have obvious routes <strong>of</strong> formation. The presence <strong>of</strong> pinic<br />
acidinthe 3 -carene/O 3 reaction system may derive from similar routes to those<br />
responsible for the formation <strong>of</strong> pinic acid in the sabinene/O 3 reaction system.<br />
7. Summary <strong>and</strong> Conclusions<br />
This study has identified a substantial fraction <strong>of</strong> the particulate products from<br />
ozone oxidation <strong>of</strong> each <strong>of</strong> the four monoterpenes: α-pinene, β-pinene, 3 -carene,<br />
<strong>and</strong> sabinene. β-Pinene <strong>and</strong> sabinene are structurally analogous in that both are<br />
bicyclic <strong>and</strong> have an external unsaturated bond where ozone oxidation takes place.<br />
α-Pinene <strong>and</strong> 3 -carene also share one common structural moiety, an internal unsaturated<br />
bond. A number <strong>of</strong> analogous product pairs, including major products,<br />
have been identified for each pair <strong>of</strong> monoterpenes. These products are consistent<br />
with the established underst<strong>and</strong>ing <strong>of</strong> the mechanism <strong>of</strong> ozone-alkene reactions.