Molecular Neurobiology - Universidad Autónoma de Madrid
Molecular Neurobiology - Universidad Autónoma de Madrid
Molecular Neurobiology - Universidad Autónoma de Madrid
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Molecular</strong> <strong>Neurobiology</strong><br />
previous next<br />
Table of contents Section contents Home Exit<br />
D1<br />
Glycine neurotransporters: molecular structure, biogenesis and regulation<br />
Research summary<br />
Our group is focused on the study of molecular mechanism, biogenesis, intracellular traffic, regulation and<br />
pharmacology of plasma membrane glycine transporters (GLYT1 and GLYT2) that play a major role in the final<br />
step of glycinergic transmission by removing specifically the neurotransmitter from the synapse. Our aim is to<br />
gain insight in the physiology and pathologies associated to alterations of glycinergic neurotransmission as<br />
neuropathic pain and muscle tone pathologies as hyperekplexia, myoclonus or epilepsia. In humans, mutations<br />
in SLC6A5 gene (GLYT2) are the cause of autosomal sporadic hyperekplexia.<br />
Research summary<br />
Staff<br />
Publications<br />
Other activities<br />
In collaboration with the CBMSO Bioinformatics group (A. Ramírez / A. Morreale) we have generated 3D<br />
mo<strong>de</strong>ls for GLYT1 and GLYT2. Using molecular dynamics simulations and electrostatic calculations of the<br />
transporters in the presence of Na + , we have i<strong>de</strong>ntified and experimentally confirmed, the residues involved<br />
in the additional Na + site (Na3) of GLYT2 (stoichiometry: 3Na + :1Cl - :1glycine). The replacement of Asp471<br />
located in TM6 of GLYT2, but not the equivalent position in GLYT1 (Asp295, coupled to two Na + ), reduced Na +<br />
affinity and cooperativity of glycine transport. An efficient allosteric communication between Asp471 and the<br />
Na1-2 sites was inferred from the differential Na + -induced responses to tiol reagents by target cysteines in 471<br />
(GLYT2) and 295 (GLYT1). Target cysteines show differential glycine-<strong>de</strong>pen<strong>de</strong>nt accessibility in both isoforms.<br />
Na + protected target cysteine from inhibition with reagent at a conformationally restricted temperature in GLYT2<br />
but not in GLYT1, indicating direct binding to position 471.<br />
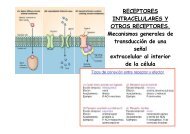
We studied the traffic of GLYT2, which recycles between endosomes and plasma membrane through<br />
constitutive and regulated pathways. The activation of PKC by phorbol-esters inhibits GLYT2 transport through<br />
an increase of the internalization rate causing net accumulation of the protein in internal compartments and a<br />
redistribution of the transporter from rafts to non-raft domains in the plasma membrane of brainstem primary<br />
neurons and synaptosomes.<br />
CBM 2007-2008