CHE 275 ALKANES AND CYCLOALKANES CHAP 3 ASSIGN ...
CHE 275 ALKANES AND CYCLOALKANES CHAP 3 ASSIGN ...
CHE 275 ALKANES AND CYCLOALKANES CHAP 3 ASSIGN ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>CHE</strong> <strong>275</strong> <strong>ALKANES</strong> <strong>AND</strong> CYCLO<strong>ALKANES</strong> <strong>CHAP</strong> 3 <strong>ASSIGN</strong><br />
COMFORMATIONS <strong>AND</strong> cis-trans STEREOISOMERS<br />
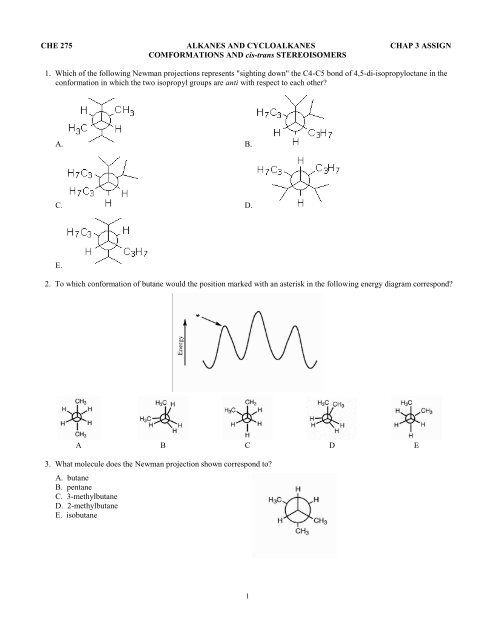
1. Which of the following Newman projections represents "sighting down" the C4-C5 bond of 4,5-di-isopropyloctane in the<br />
conformation in which the two isopropyl groups are anti with respect to each other?<br />
A. B.<br />
C. D.<br />
E.<br />
2. To which conformation of butane would the position marked with an asterisk in the following energy diagram correspond?<br />
A B C D E<br />
3. What molecule does the Newman projection shown correspond to?<br />
A. butane<br />
B. pentane<br />
C. 3-methylbutane<br />
D. 2-methylbutane<br />
E. isobutane<br />
1
4. Which of the following Newman Projections represents the lowest energy conformation for butane?<br />
A. B.<br />
C. D.<br />
E.<br />
5. Choose the zigzag structure that corresponds to the molecule depicted in the following Newman projection.<br />
A B C D E<br />
6. Which of the following would be the Newman projection of a C-C bond in chair cyclohexane?<br />
A. B.<br />
C. D.<br />
E.<br />
2
7. Which of the following isomers of the formula C 8 H 14 would you expect to give the highest heat of combustion?<br />
A. B. C. D. E.<br />
8. What would be the best name for the following compound? (Neglect any cis-trans isomerism that is possible.)<br />
A. 1-ethyl-3,4-dimethylcyclohexane<br />
B. 3-ethyl-1,6-dimethylcyclohexane<br />
C. 1-ethyl-4,5-dimethylcyclohexane<br />
D. 5-ethyl-1,2-dimethylcyclohexane<br />
E. 4-ethyl-1,2-dimethylcyclohexane<br />
9. Which of the following cyclic alkanes is the most stable (i.e., lowest energy)?<br />
A. B. C. D. E.<br />
10. What is the lowest energy stereoisomer of cis-1-isopropyl-3-methylcyclohexane?<br />
A. B.<br />
C. D.<br />
E.<br />
11. Which of the following most accurately illustrates the correct geometry of the axial and equatorial bonds in the chair<br />
conformation of cyclohexane?<br />
A B C D E<br />
12. What is the correct IUPAC name for the following molecule?<br />
A. trans-3-methyl-5-ethylcyclohexane<br />
B. cis-3-methyl-5-ethylcyclohexane<br />
C. trans-1-ethyl-3-methylcyclohexane<br />
D. cis-1-ethyl-3-methylcyclohexane<br />
E. trans-1-methyl-3-ethylcyclohexane<br />
13. What is the most stable conformation of trans-1-tert -butyl-2-methylcyclohexane?<br />
A B C D E<br />
3
14. In the following chair conformation of methylcyclohexane, which hydrogen (indicated by arrows) causes the greatest steric<br />
strain with the axial methyl group? This conformation (with the methyl group occupying an axial orientation) is less stable<br />
than the conformation in which the methyl group is equatorial.<br />
A. B.<br />
C. D.<br />
E.<br />
15. What is the most stable conformation of cis -1-sec-butyl-4-methylcyclohexane?<br />
A B C D E<br />
16. How many axial hydrogens are present in the following molecule?<br />
A. two<br />
B. three<br />
C. four<br />
D. five<br />
E. six<br />
17. Cyclobutane is an example of a molecule that shows<br />
A. only angle strain B. 109.5° bond angles<br />
C. angle and torsional strain D. only torsional strain<br />
E. a low energy ring structure<br />
18. Consider the graph of total strain energy versus ring size. Which of the following statements is false?<br />
A. Among the smaller ring sizes, six-membered rings are the most stable.<br />
B. Medium-sized rings (7-11 carbons) are less stable than larger rings.<br />
C. Five-membered rings have about the same strain energy as seven-membered rings.<br />
D. Four-membered rings are significantly more stable than three-membered rings.<br />
E. Ten-membered rings are the least stable of the medium-sized rings (7-11 carbons).<br />
4
19. Which of the following definitions most accurately describes stereo-isomers?<br />
A. compounds that differ from one another by one bond rotations<br />
B. compounds that differ from one another as a result of differences in spatial orientation that are caused by altering their<br />
connectivity (one structure compared with the other)<br />
C. compounds that have exactly the same connectivity but differ in the arrangement of their atoms in space<br />
D. compounds that are very similar in structure but contain several different functional groups<br />
E. compounds that have the same number of carbon atoms but differ because one of them is acyclic (no ring) and the other<br />
is cyclic (contains a ring)<br />
20. Which one of the following statements is incorrect?<br />
A. Cyclopropane has a higher heat of combustion than does cyclohexane.<br />
B. Cyclopentane is destabilized somewhat by tortional (eclipsing) interactions.<br />
C. Cyclohexane exists predominantly in an all-staggered conformation.<br />
D. Cyclopropane is destabilized by both angle strain and tortional (eclipsing) interactions.<br />
E. Cyclobutane has a higher heat of combustion per CH 2 group than does cyclohexane.<br />
21. Which conformation of cis -1-isopropyl-4-methylcyclohexane would be of the lowest energy?<br />
A. B.<br />
C. D.<br />
E.<br />
22. Cyclopentane exhibits more torsional strain than cyclohexane because,<br />
A. cyclopentane exists predominantly in the boat form.<br />
B. its atoms are in a partially eclipsed conformation.<br />
C. its atoms are in the staggered conformation.<br />
D. its bond angels deviate greatly from 109°.<br />
23. trans-1,2-Dibromocyclohexane is represented by structure(s):<br />
A. I<br />
B. II<br />
C. III<br />
D. II and III<br />
E. I and II<br />
24. What is the correct name of the following compound?<br />
A. 1-Chlorobicyclo[4.1.1]octane<br />
B. 2-Chlorobicyclo[4.1.0]octane<br />
C. 2-Chlorobicyclo[4.1.1]octane<br />
D. 2-Chlorobicyclo[4.1.1]heptane<br />
E. 5-Chlorobicyclo[4.1.1]octane<br />
Br<br />
H<br />
Br<br />
H<br />
H<br />
Br<br />
Br<br />
H<br />
H<br />
I II III<br />
Cl<br />
Br<br />
Br<br />
H<br />
5
25. Which of the following is bicyclo[3.2.2]nonane?<br />
A. I<br />
B. II<br />
C. III<br />
D. IV<br />
E. V<br />
I II III<br />
26. cis-1,3-Dibromocyclohexane is represented by structure(s):<br />
A. I<br />
B. II<br />
C. III<br />
D. II and III<br />
E. I and II<br />
Br<br />
H<br />
IV V<br />
Br<br />
Br<br />
H<br />
H<br />
Br<br />
H H<br />
Br<br />
Br<br />
H<br />
I II III<br />
27. The preferred conformation of cis-3-tert-butyl-1-methylcyclohexane is the one in which:<br />
A. the tert-butyl group is axial and the methyl group is equatorial.<br />
B. the methyl group is axial and the tert-butyl group is equatorial.<br />
C. both groups are axial.<br />
D. both groups are equatorial.<br />
E. the molecule exists in a boat conformation.<br />
28. Which of the following can be described as cis isomers?<br />
A. I<br />
B. II, V<br />
C. III, IV<br />
D. I, III and IV<br />
E. None of the above are cis isomers.<br />
HO<br />
Br Cl<br />
Br<br />
CH 3<br />
F<br />
I II III<br />
CH<br />
CH 3<br />
3<br />
HO<br />
CH 2 CH 3<br />
IV V<br />
29. Which of the following will have the same energy after undergoing ring flip?<br />
A. I<br />
B. II<br />
C. III<br />
D. IV<br />
E. V<br />
HO<br />
OH<br />
Cl<br />
CH 3<br />
I II III<br />
F<br />
Br<br />
HO<br />
CH 3<br />
CH 2 CH 3<br />
CH 2 CH 3<br />
IV V<br />
6
30. Which of these C 10 H 18 isomers is predicted to be the most stable?<br />
A. I<br />
B. II<br />
C. III<br />
D. IV<br />
E. V I II III<br />
H<br />
H<br />
H<br />
IV<br />
H<br />
V<br />
EXTRA CREDIT<br />
31. The most stable conformation for 1,2-ethanediol (ethylene glycol) is shown below. It is the most stable conformation<br />
because:<br />
A. this corresponds to an anti conformation.<br />
B. in general, gauche conformations possess the minimum energy.<br />
C. it is stabilized by intramolecular hydrogen bonding.<br />
D. it is a staggered conformation.<br />
E. it has the highest energy of all the possibilities.<br />
H<br />
H<br />
H<br />
H<br />
OH<br />
OH<br />
32. The most stable conformation of 2,3-dibromobutane, viewed through the C-2—C-3 bond :<br />
A. I<br />
B. II<br />
C. III<br />
D. IV<br />
E. V<br />
Br<br />
Br<br />
CH 3<br />
H<br />
H<br />
H<br />
H 3 C Br<br />
H<br />
Br<br />
H 3 C Br<br />
CH 3<br />
H<br />
I II III<br />
H 3 C<br />
Br<br />
H<br />
H<br />
Br<br />
H 3 C<br />
Br<br />
H<br />
Br<br />
CH 3<br />
H 3 C<br />
H H 3 C<br />
Br<br />
CH 3<br />
IV V<br />
H<br />
7
NAME_______________________________________<br />
DATE______________<br />
ANSWER SHEET<br />
<strong>CHE</strong> <strong>275</strong> – <strong>CHAP</strong> 3 <strong>ASSIGN</strong><br />
1. _______________ 11. _______________ 21. _______________ 31. _______________<br />
2. _______________ 12. _______________ 22. _______________ 32. _______________<br />
3. _______________ 13. _______________ 23. _______________ 33. _______________<br />
4. _______________ 14. _______________ 24. _______________ 34. _______________<br />
5. _______________ 15. _______________ 25. _______________ 35. _______________<br />
6. _______________ 16. _______________ 26. _______________ 36. _______________<br />
7. _______________ 17. _______________ 27. _______________ 37. _______________<br />
8. _______________ 18. _______________ 28. _______________ 38. _______________<br />
9. _______________ 19. _______________ 29. _______________ 39. _______________<br />
10. _______________ 20. _______________ 30. _______________ 40. _______________<br />
SSI 2013<br />
8