Nanomedicine - European Science Foundation
Nanomedicine - European Science Foundation
Nanomedicine - European Science Foundation
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
25<br />
3. <strong>European</strong> Situation and Forward Look –<br />
SWOT Analysis<br />
All the technology based workshops held in Amsterdam from 1 to 5 March 2004 were independently invited to<br />
conduct a SWOT analysis regarding the current status of <strong>European</strong> activities in the field. There was considerable<br />
consensus across these groups and the results were summarised and presented at the Consensus Conference<br />
held at Le Bischenberg. Below is a summary of the most significant points agreed.<br />
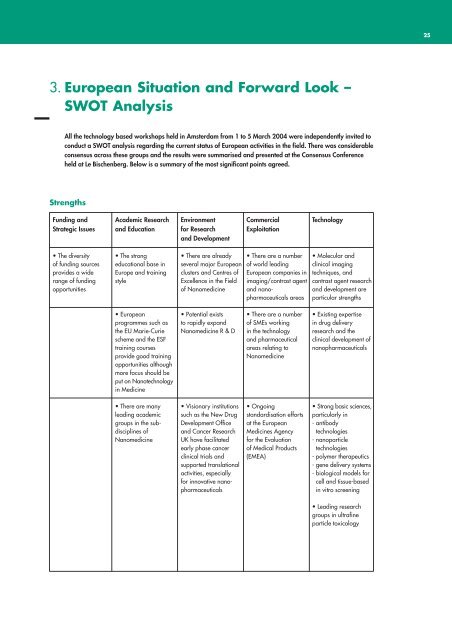
Strengths<br />
Funding and<br />
Strategic Issues<br />
Academic Research<br />
and Education<br />
Environment<br />
for Research<br />
and Development<br />
Commercial<br />
Exploitation<br />
Technology<br />
• The diversity<br />
of funding sources<br />
provides a wide<br />
range of funding<br />
opportunities<br />
• The strong<br />
educational base in<br />
Europe and training<br />
style<br />
• There are already<br />
several major <strong>European</strong><br />
clusters and Centres of<br />
Excellence in the Field<br />
of <strong>Nanomedicine</strong><br />
• There are a number<br />
of world leading<br />
<strong>European</strong> companies in<br />
imaging/contrast agent<br />
and nanopharmaceuticals<br />
areas<br />
• Molecular and<br />
clinical imaging<br />
techniques, and<br />
contrast agent research<br />
and development are<br />
particular strengths<br />
• <strong>European</strong><br />
programmes such as<br />
the EU Marie-Curie<br />
scheme and the ESF<br />
training courses<br />
provide good training<br />
opportunities although<br />
more focus should be<br />
put on Nanotechnology<br />
in Medicine<br />
• Potential exists<br />
to rapidly expand<br />
<strong>Nanomedicine</strong> R & D<br />
• There are a number<br />
of SMEs working<br />
in the technology<br />
and pharmaceutical<br />
areas relating to<br />
<strong>Nanomedicine</strong><br />
• Existing expertise<br />
in drug delivery<br />
research and the<br />
clinical development of<br />
nanopharmaceuticals<br />
• There are many<br />
leading academic<br />
groups in the subdisciplines<br />
of<br />
<strong>Nanomedicine</strong><br />
• Visionary institutions<br />
such as the New Drug<br />
Development Office<br />
and Cancer Research<br />
UK have facilitated<br />
early phase cancer<br />
clinical trials and<br />
supported translational<br />
activities, especially<br />
for innovative nanopharmaceuticals<br />
• Ongoing<br />
standardisation efforts<br />
at the <strong>European</strong><br />
Medicines Agency<br />
for the Evaluation<br />
of Medical Products<br />
(EMEA)<br />
• Strong basic sciences,<br />
particularly in<br />
- antibody<br />
technologies<br />
- nanoparticle<br />
technologies<br />
- polymer therapeutics<br />
- gene delivery systems<br />
- biological models for<br />
cell and tissue-based<br />
in vitro screening<br />
• Leading research<br />
groups in ultrafine<br />
particle toxicology