Lesson 32 Mineral Cycling - Alaska Geobotany Center
Lesson 32 Mineral Cycling - Alaska Geobotany Center
Lesson 32 Mineral Cycling - Alaska Geobotany Center
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
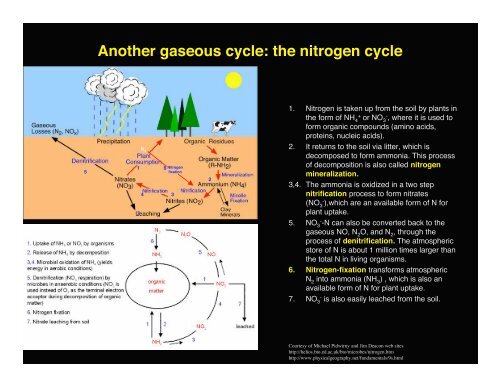
Another gaseous cycle: the nitrogen cycle<br />
5<br />
N 2<br />
1<br />
4<br />
7<br />
6<br />
Nitrogen<br />
fixation<br />
3<br />
2<br />
1. Nitrogen is taken up from the soil by plants in<br />
theformofNH 4 + or NO 3 - , where it is used to<br />
form organic compounds (amino acids,<br />
proteins, nucleic acids).<br />
2. It returns to the soil via litter, which is<br />
decomposed to form ammonia. This process<br />
of decomposition is also called nitrogen<br />
mineralization.<br />
3,4. The ammonia is oxidized in a two step<br />
nitrification process to form nitrates<br />
(NO 3 - ),which are an available form of N for<br />
plant uptake.<br />
5. NO 3 - -N can also be converted back to the<br />
gaseous NO, N 2 O, and N 2 , through the<br />
process of denitrification. The atmospheric<br />
store of N is about 1 million times larger than<br />
the total N in living organisms.<br />
6. Nitrogen-fixation transforms atmospheric<br />
N 2 into ammonia (NH 3 ) , which is also an<br />
available form of N for plant uptake.<br />
7. NO 3 - is also easily leached from the soil.<br />
Courtesy of Michael Pidwirny and Jim Deacon web sites<br />
http://helios.bto.ed.ac.uk/bto/microbes/nitrogen.htm<br />
http://www.physicalgeography.net/fundamentals/9s.html