LOMOPRIM DS Tablet - Lomus Pharmaceuticals Pvt. Ltd.
LOMOPRIM DS Tablet - Lomus Pharmaceuticals Pvt. Ltd.
LOMOPRIM DS Tablet - Lomus Pharmaceuticals Pvt. Ltd.
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
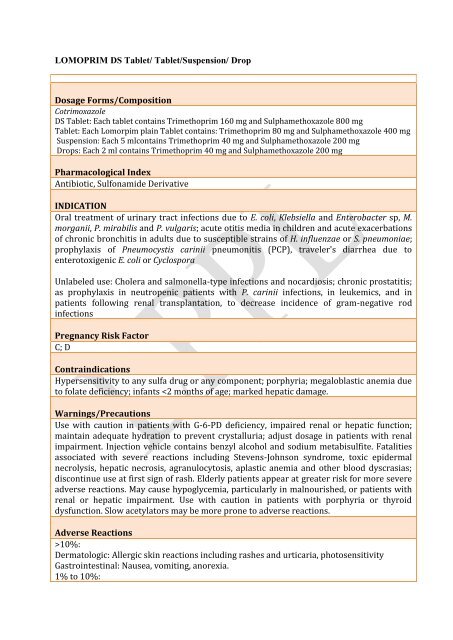
<strong>LOMOPRIM</strong> <strong>DS</strong> <strong>Tablet</strong>/ <strong>Tablet</strong>/Suspension/ Drop<br />
Dosage Forms/Composition<br />
Cotrimoxazole<br />
<strong>DS</strong> <strong>Tablet</strong>: Each tablet contains Trimethoprim 160 mg and Sulphamethoxazole 800 mg<br />
<strong>Tablet</strong>: Each Lomorpim plain <strong>Tablet</strong> contains: Trimethoprim 80 mg and Sulphamethoxazole 400 mg<br />
Suspension: Each 5 mlcontains Trimethoprim 40 mg and Sulphamethoxazole 200 mg<br />
Drops: Each 2 ml contains Trimethoprim 40 mg and Sulphamethoxazole 200 mg<br />
Pharmacological Index<br />
Antibiotic, Sulfonamide Derivative<br />
INDICATION<br />
Oral treatment of urinary tract infections due to E. coli, Klebsiella and Enterobacter sp, M.<br />
morganii, P. mirabilis and P. vulgaris; acute otitis media in children and acute exacerbations<br />
of chronic bronchitis in adults due to susceptible strains of H. influenzae or S. pneumoniae;<br />
prophylaxis of Pneumocystis carinii pneumonitis (PCP), traveler's diarrhea due to<br />
enterotoxigenic E. coli or Cyclospora<br />
Unlabeled use: Cholera and salmonella-type infections and nocardiosis; chronic prostatitis;<br />
as prophylaxis in neutropenic patients with P. carinii infections, in leukemics, and in<br />
patients following renal transplantation, to decrease incidence of gram-negative rod<br />
infections<br />
Pregnancy Risk Factor<br />
C; D<br />
Contraindications<br />
Hypersensitivity to any sulfa drug or any component; porphyria; megaloblastic anemia due<br />
to folate deficiency; infants 10%:<br />
Dermatologic: Allergic skin reactions including rashes and urticaria, photosensitivity<br />
Gastrointestinal: Nausea, vomiting, anorexia.<br />
1% to 10%:
Dermatologic: Stevens-Johnson syndrome, toxic epidermal necrolysis (rare).<br />
Hematologic: Blood dyscrasias.<br />
Hepatic: Hepatitis .<br />
per kg body mass daily which approximately corresponds to the following dosage according to age:<br />
Children 6 - 12 years: Two medicine measures (10 mL) twice daily.<br />
In the treatment of acute infections, co-trimoxazole should be administered for at least 5 days or for<br />
at least 2 days after the symptoms have disappeared. If clinical improvement is not evident after<br />
7 days therapy, the patient should be reassessed.<br />
Patient Information<br />
Take oral medication with 8 oz of water on an empty stomach (1 hour before or 2 hours<br />
after meals) for best absorption. Finish all medication; do not skip doses. You may<br />
experience increased sensitivity to sunlight; use sunblock, wear protective clothing and<br />
dark glasses, or avoid direct exposure to sunlight. Small frequent meals, frequent mouth<br />
care, sucking lozenges, or chewing gum may reduce nausea or vomiting. Report skin rash,<br />
sore throat, blackened stool, or unusual bruising or bleeding immediately. Pregnancy<br />
precautions: Inform prescriber if you are or intend to be pregnant.<br />
LOMUS Drug Information Center<br />
<strong>Lomus</strong> <strong>Pharmaceuticals</strong> <strong>Pvt</strong>. <strong>Ltd</strong>.<br />
P.O. Box No 4506, <strong>Lomus</strong> House (Corporate office),<br />
Kailash Chour, Lazimpat, Kathmandu, Nepal<br />
Ph: 4436396 (Hunting Line). Fx: 977-1-4436395<br />
E-mail: druginfo@lomus.com.np