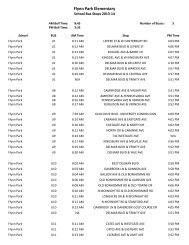
AP Bio Lab 3--Practicing with 3-D Protein Structure Directions: In this ...
AP Bio Lab 3--Practicing with 3-D Protein Structure Directions: In this ...
AP Bio Lab 3--Practicing with 3-D Protein Structure Directions: In this ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Name__________________________________________<br />
Date______________________<br />
<strong>AP</strong> <strong>Bio</strong> <strong>Lab</strong> 3--<strong>Practicing</strong> <strong>with</strong> 3-D <strong>Protein</strong> <strong>Structure</strong><br />
<strong>Directions</strong>: <strong>In</strong> <strong>this</strong> lab we will use strips of paper to simulate polypeptides. We will manipulate the paper to demonstrate<br />
the four levels of protein structure.<br />
1. Primary <strong>Structure</strong>:<br />
<br />
<br />
<br />
<br />
<br />
What determines the primary structure of a protein?__________________________________________________<br />
What are the monomers in a polypeptide called?____________________________________________________<br />
What two functional groups are present in all monomers?_____________________________________________<br />
How many common monomers exist?____________________________________________________________<br />
Draw the basic structural formula for an amino acid in the box. <strong>Lab</strong>el the parts of the molecule in the diagram.<br />
The “R” groups can have several<br />
properties, list them below:<br />
_______________________________<br />
_______________________________<br />
_______________________________<br />
<br />
What is the name of the bond formed between 2 amino acids? Draw <strong>this</strong> bond below.<br />
What molecule is released by the<br />
What is the name of the molecule<br />
formation of the bond shown here? formed by one of these bonds?<br />
_______________ __________________________
Name__________________________________________<br />
Date______________________<br />
2. Secondary <strong>Structure</strong>:<br />
Follow the directions below to assemble your chain of amino acids into a secondary structure. Cut out the two<br />
rectangles on the next page cutting along the solid black lines. Fold one rectangle, from the short side into a pleat. After<br />
folding, cut carefully along the dotted black line. Cut the other rectangle carefully at the dotted lines. Using your pen or<br />
pencil, carefully wrap the paper around your pen/pencil to form a coil. Try not to rip the paper.<br />
<br />
What is the name of the secondary structure that looks like a coil?_______________________________<br />
What is the name of the other secondary structure?___________________________________________<br />
<br />
Both of these structures are formed by the hydrogen bonds that form between adjacent chains. <strong>In</strong> the box below<br />
draw <strong>this</strong> interaction (The double bonded O in the peptide bond interacts <strong>with</strong> the H on the amino group of an adjacent<br />
peptide bond)
Name__________________________________________<br />
Date______________________<br />
Part 3: Tertiary structure: After building the secondary structures, wrap your polypeptide into a glob. This represents<br />
tertiary structure.<br />
<br />
<br />
<br />
<br />
<br />
What is the primary cause of tertiary structure?______________________________________________________<br />
Two side chains containing a sulfhydryl group can interact to make strong, covalent_________________________<br />
A side chain containing a positively charged atom will form and _______________________ bond <strong>with</strong> a side chain<br />
containing a ________________________ charged atom.<br />
Hydrogen atoms involved in polar bonds will form _____________________ bonds <strong>with</strong> oxygen or nitrogen atoms.<br />
Part 4: Quaternary structure.<br />
<br />
<br />
<br />
<br />
<br />
Place the two chains of polypeptides together into a complex. This represents the final shape of a protein.<br />
What interactions will contribute to the quaternary structure?___________________________________________<br />
The final shape of a protein determines its ___________________________. A single amino acid that is<br />
incorrectly used in the making of a protein can result in death.<br />
<strong>Protein</strong>s main roles in living things are: _________________________, __________________________<br />
N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-<br />
C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-<br />
C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-<br />
N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-<br />
C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-<br />
C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-N