Acid-Base Web Investigation - University City Schools
Acid-Base Web Investigation - University City Schools
Acid-Base Web Investigation - University City Schools
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
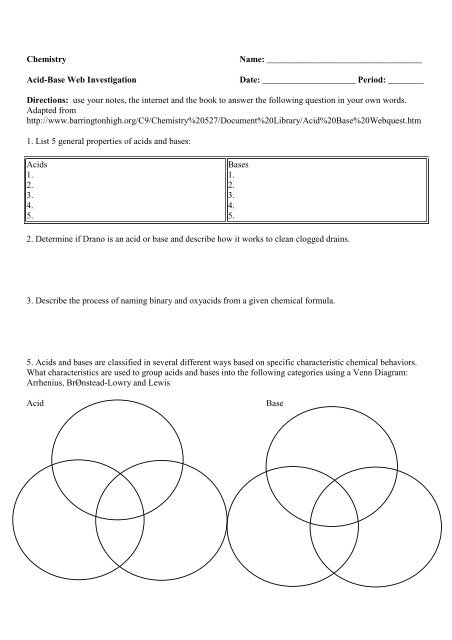
Chemistry<strong>Acid</strong>-<strong>Base</strong> <strong>Web</strong> <strong>Investigation</strong>Name: ___________________________________Date: _____________________ Period: ________Directions: use your notes, the internet and the book to answer the following question in your own words.Adapted fromhttp://www.barringtonhigh.org/C9/Chemistry%20527/Document%20Library/<strong>Acid</strong>%20<strong>Base</strong>%20<strong>Web</strong>quest.htm1. List 5 general properties of acids and bases:<strong>Acid</strong>s1.2.3.4.5.<strong>Base</strong>s1.2.3.4.5.2. Determine if Drano is an acid or base and describe how it works to clean clogged drains.3. Describe the process of naming binary and oxyacids from a given chemical formula.5. <strong>Acid</strong>s and bases are classified in several different ways based on specific characteristic chemical behaviors.What characteristics are used to group acids and bases into the following categories using a Venn Diagram:Arrhenius, BrØnstead-Lowry and Lewis<strong>Acid</strong><strong>Base</strong>
12. How might you use an acid-base indicator to determine if the pH of the water in your fish tank was suitablefor your pet fish? Should the environment for fish be acidic, basic, or neutral? What indicator might you use todetermine if your tank is suitable? How might a tritration be useful in this process?13. The concentration (molarity) of an acid solution is not the same as the strength of the acid. What is thedifference?