ANTIFREEZE PROTEINS - RCSB Protein Data Bank
ANTIFREEZE PROTEINS - RCSB Protein Data Bank
ANTIFREEZE PROTEINS - RCSB Protein Data Bank
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
MOLECULE OF THE MONTH:<br />
www.pdb.org<br />
info@rcsb.org<br />
<strong>ANTIFREEZE</strong> <strong>PROTEINS</strong><br />
10.2210/rcsb_pdb/mom_2009_12<br />
Ice is a big problem for<br />
organisms that live in cold<br />
climates. Once the temperature<br />
dips below freezing, ice<br />
crystals steadily grow and<br />
burst cells. This danger,<br />
however, has not limited the<br />
spread of life on Earth to<br />
temperate regions.<br />
Organisms of all types–<br />
plants, animals, fungi and<br />
bacteria–have developed<br />
ways to combat the deadly<br />
growth of ice crystals. In<br />
some cases, they pack their<br />
cells with small antifreeze<br />
compounds like sugars or<br />
glycerol. But in cases where<br />
extra help is needed, cells<br />
make specialized antifreeze<br />
proteins to protect<br />
themselves as the<br />
temperature drops.<br />
About the<br />
<strong>RCSB</strong> PDB Molecule of the Month<br />
Using selected molecules from the PDB<br />
archive, each feature includes an<br />
introduction to the structure and function<br />
of the molecule, a discussion of its<br />
relevance to human health and welfare,<br />
and suggestions for viewing and<br />
accessing further details.<br />
The <strong>RCSB</strong> PDB Molecule of the Month<br />
is read by students, teachers, and scientists<br />
worldwide at www.pdb.org.<br />
This December 2009 edition was<br />
written and illustrated by<br />
David S. Goodsell<br />
(<strong>RCSB</strong> PDB and The Scripps<br />
Research Institute).<br />
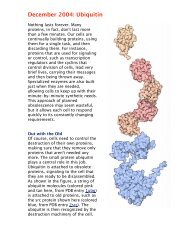
1kdf<br />
Nice Ice<br />
Antifreeze proteins don't stop the growth of ice<br />
crystals, but they limit the growth to manageable<br />
sizes. For this reason, they are also known<br />
as ice-restructuring proteins. This is necessary<br />
because of an unusual property of ice called<br />
recrystallization. When water begins to freeze,<br />
many small crystals form, but then a few small<br />
crystals dominate and grow larger and larger,<br />
stealing water molecules from the surrounding<br />
small crystals. Antifreeze proteins counteract<br />
this recrystallization effect. They bind to the<br />
surface of the small ice crystals and slow or prevent<br />
the growth into larger dangerous crystals.<br />
Supercooling<br />
Antifreeze proteins lower the freezing point of<br />
water by a few degrees, but surprisingly, they<br />
don't change the melting point. This process of<br />
depressing the freezing point while not effecting<br />
the melting point is termed thermal hysteresis.<br />
The most effective antifreeze proteins<br />
are made by insects, which lower the freezing<br />
point by about 6 degrees. However, antifreeze<br />
proteins, even the ones from plants and bacteria<br />
that have smaller effects on freezing point,<br />
are useful in another way. They are placed outside<br />
cells where they control the size of ice crystals<br />
and prevent catastrophic ice crystal formation<br />
when the temperature drops below the<br />
(lowered) freezing point.<br />
Icy Ice Cream<br />
Antifreeze proteins have been useful in industry.<br />
For instance, natural antifreeze proteins<br />
purified from cold-water ocean pout (shown<br />
here from PDB entry 1kdf) have been used as<br />
a preservative in ice cream. They coat the fine<br />
ice crystals that give ice cream its smooth texture,<br />
and prevent it from recrystallizing<br />
during storage and delivery into chunky, icy<br />
ice cream. Researchers are also experimenting<br />
with antifreeze proteins as a way to preserve<br />
tissues and organs that are stored at low<br />
temperatures, reducing the possible damage<br />
from ice crystals.
<strong>ANTIFREEZE</strong> <strong>PROTEINS</strong><br />
<strong>RCSB</strong> <strong>Protein</strong> <strong>Data</strong> <strong>Bank</strong><br />
The <strong>Protein</strong> <strong>Data</strong> <strong>Bank</strong> (PDB) is the<br />
single worldwide repository for the<br />
processing and distribution of 3D<br />
structure data of large molecules of<br />
proteins and nucleic acids. The <strong>RCSB</strong><br />
PDB is operated by Rutgers, The State<br />
University of New Jersey and the San<br />
Diego Supercomputer Center and the<br />
Skaggs School of Pharmacy and<br />
Pharmaceutical Sciences at the University<br />
of California, San Diego–two members<br />
of the Research Collaboratory for<br />
Structural Bioinformatics (<strong>RCSB</strong>).<br />
It is supported by funds from the<br />
National Science Foundation, the<br />
National Institute of General Medical<br />
Sciences, the Office of Science,<br />
Department of Energy, the National<br />
Library of Medicine, the National<br />
Cancer Institute, the National Institute<br />
of Neurological Disorders and Stroke<br />
and the National Institute of Diabetes<br />
& Digestive & Kidney Diseases.<br />
The <strong>RCSB</strong> PDB is a member of<br />
the worldwide PDB<br />
(wwPDB; www.wwpdb.org).<br />
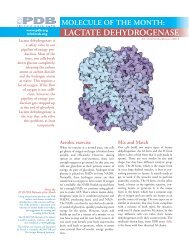
1kdf 1wfb 1ezg 1eww 2pne<br />
ocean pout<br />
winter flounder<br />
Many Solutions to the<br />
Same Problem<br />
Antifreeze proteins are a perfect example of<br />
convergent evolution. Looking at the proteins<br />
used by different organisms, we see that many<br />
different proteins have been selected to serve<br />
this same function. Several examples are<br />
included above. All of these are small proteins<br />
with a flat surface that is rich in threonine<br />
(colored lighter blue here), which binds to the<br />
surface of ice crystals. These include two proteins<br />
from fish, the ocean pout (1kdf) and the<br />
winter flounder (1wfb), and three very active<br />
proteins from insects, the yellow mealworm<br />
beetle (1ezg), the spruce budworm moth<br />
(1eww), and the snow flea (2pne).<br />
yellow<br />
mealworm<br />
beettle<br />
spruce<br />
budworm<br />
moth<br />
snow flea<br />
Exploring the Structure<br />
Antifreeze proteins bind to ice crystals, blocking<br />
the surface and preventing growth of the<br />
crystal. The structure of snow flea antifreeze<br />
protein (2pne) will give you an idea of what<br />
this recognition may be like. In the crystal<br />
structure, the ice-binding surface of the protein<br />
is covered with strings of water molecules<br />
(shown here in red). These water molecules are<br />
spaced similarly to the water molecules in ice<br />
crystals. So you can imagine this protein binding<br />
to the geometric lattice of water molecules<br />
in ice in a similar way.<br />
References:<br />
1kdf: F.D. Sonnichsen, C.I. DeLuca, P.L. Davies,<br />
B.D. Sykes (1996) Refined solution structure of type<br />
III antifreeze protein: hydrophobic groups may be<br />
involved in the energetics of the protein-ice interaction.<br />
Structure 4: 1325-1337<br />
1wfb: F. Sicheri, D.S. Yang (1995) Ice-binding structure<br />
and mechanism of an antifreeze protein from<br />
winter flounder. Nature 375: 427-431<br />
1ezg: Y.C. Liou, A. Tocilj, P.L. Davies, Z. Jia (2000)<br />
Mimicry of ice structure by surface hydroxyls and<br />
water of a beta-helix antifreeze protein. Nature 406:<br />
322-324<br />
1eww: S.P. Graether, M.J. Kuiper, S.M., Gagne, V.K.<br />
Walker, Z. Jia, B.D. Sykes, P.L. Davies (2000) Betahelix<br />
structure and ice-binding properties of a hyperactive<br />
antifreeze protein from an insect. Nature 406:<br />
325-328<br />
2pne: B.L. Pentelute, Z.P. Gates, V. Tereshko, J.L.<br />
Dashnau, J.M. Vanderkooi, A.A. Kossiakoff, S.B.<br />
Kent (2008) X-ray structure of snow flea antifreeze<br />
protein determined by racemic crystallization of synthetic<br />
protein enantiomers. J.Am.Chem.Soc. 130:<br />
9695-9701<br />
Topics for Further Exploration<br />
1. Antifreeze proteins are examples of convergent evolution.<br />
Can you find other examples in the PDB where two entirely<br />
different proteins perform the same function?<br />
2. The insect antifreeze proteins are examples of solenoidal<br />
folds, where the protein chain loops around like a spring.<br />
Compare the way the chain is folded in the beetle and<br />
moth proteins with the entirely different type of looping<br />
fold in the snow flea protein. Can you find other examples<br />
of solenoidal folds in the PDB (hint: look at the SCOP<br />
classification of these proteins, available at the bottom of<br />
the structure browser page).<br />
Additional Information on Antifreeze <strong>Protein</strong>s<br />
• S. Venkatesh and C. Dayananda (2008) Properties, potentials, and prospects of antifreeze proteins. Critical Reviews in Biotechnology<br />
28: 57-82.<br />
• A. Regand and H. D. Goff (2006) Ice recrystallization inhibition in ice cream as affected by ice restructuring proteins from winter<br />
wheat grass. Journal of Dairy Science 89: 49-57.<br />
• Z. Jia and P. L. Davies (2002) Antifreeze proteins: an unusual receptor-ligand interaction. Trends in Biochemical Sciences 27: 101-106.