December 2004: Ubiquitin
December 2004: Ubiquitin
December 2004: Ubiquitin
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
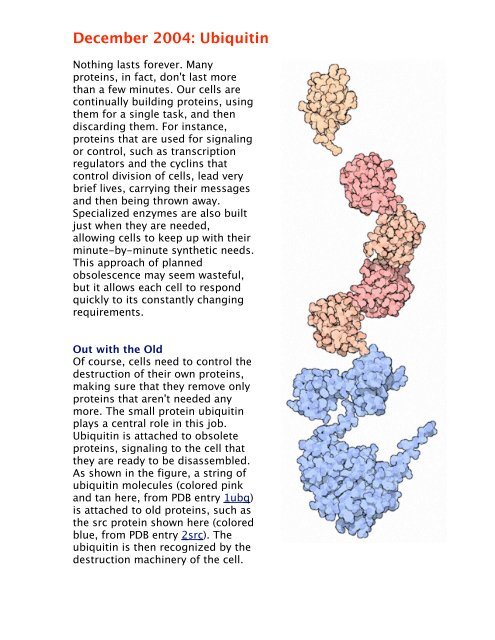
<strong>December</strong> <strong>2004</strong>: <strong>Ubiquitin</strong>Nothing lasts forever. Manyproteins, in fact, don't last morethan a few minutes. Our cells arecontinually building proteins, usingthem for a single task, and thendiscarding them. For instance,proteins that are used for signalingor control, such as transcriptionregulators and the cyclins thatcontrol division of cells, lead verybrief lives, carrying their messagesand then being thrown away.Specialized enzymes are also builtjust when they are needed,allowing cells to keep up with theirminute-by-minute synthetic needs.This approach of plannedobsolescence may seem wasteful,but it allows each cell to respondquickly to its constantly changingrequirements.Out with the OldOf course, cells need to control thedestruction of their own proteins,making sure that they remove onlyproteins that aren't needed anymore. The small protein ubiquitinplays a central role in this job.<strong>Ubiquitin</strong> is attached to obsoleteproteins, signaling to the cell thatthey are ready to be disassembled.As shown in the figure, a string ofubiquitin molecules (colored pinkand tan here, from PDB entry 1ubq )is attached to old proteins, such asthe src protein shown here (coloredblue, from PDB entry 2src ). Theubiquitin is then recognized by thedestruction machinery of the cell.
<strong>December</strong> <strong>2004</strong>: <strong>Ubiquitin</strong>Ubiquitous <strong>Ubiquitin</strong>As its name implies, ubiquitin is found in all eukaryotic cells and incells throughout your body. The Nobel Prize in Chemistry wasawarded this year to the three researchers who discovered itsessential function in 1980. In the subsequent years, it has becomeapparent that apart from its role in protein disposal, ubiquitin isalso used for other tasks, such as directing the transport ofproteins in and out of the cell. By connecting ubiquitin together inshort or long chains, or using different types of linkages betweenthe molecules, many different signals may be encoded. Because ofthe important roles it plays, ubiquitin has changed very little overthe evolution of life, so you can find a similar form in yeast cells,plant cells, and in our own cells.<strong>Ubiquitin</strong>ationThe tricky part of this wholeprocess is making sure thatubiquitin is attached only to theproper proteins. Severalspecialized enzymes sortthrough the proteins in the celland pick only the right ones.There are three types of theseenzymes, called E1, E2, and E3.The E1 enzyme, shown at thetop here from PDB entry 1r4n ,is the ubiquitin-activatingenzyme that starts the process.Powered by ATP (shown in red), it attaches the tail end of ubiquitin(shown here in light orange) to one of its own cysteine amino acids(shown in green--note, in this structure, the cysteine is mutated toalanine). Then, E1 passes the activated ubiquitin to one of severalE2 enzymes, the ubiquitin-conjugating enzymes, shown here fromPDB entry 1fxt . These E2 enzymes then work with a large number ofdifferent E3 enzymes to recognize obsolete proteins and attach theubiquitin to them. The E3 enzyme shown here, from PDB entries1ldk and 1fqv , is shaped like a big clamp. The target protein bindsin the gap (shown with the star). The left side of the enzymerecognizes the protein and the right side positions E2 to allowtransfer of its ubiquitin.
<strong>December</strong> <strong>2004</strong>: <strong>Ubiquitin</strong>Total DestructionOnce obsolete proteins are tagged with at least four ubiquitinmolecules, they are destroyed by proteasomes. Proteasomes arevoracious protein shredders, but the destructive machinery iscarefully protected so that it can't attack all of the normal proteinsin the cell. The proteasome, shown here from PDB entry 1fnt , isshaped like a cylinder, with its active sites sheltered inside the tube.The caps on the ends regulate entry into the destructive chamber,where the protein is chopped into pieces 3 to 23 amino acids long.
<strong>December</strong> <strong>2004</strong>: <strong>Ubiquitin</strong>Exploring the StructurePDB entry 1f9j shows how ubiquitin molecules are strung togetherinto chains. The crystal was grown with tetraubiquitin (four proteinsstrung together), but the connection was only observed betweentwo ubiquitin molecules in the structure. If you look closely, you cansee the unusual connection between the last glycine amino acid inchain A and the sidechain of lysine number 148 in the middle ofchain B.