One micron magnetic beads optimised for automated immunoassays
One micron magnetic beads optimised for automated immunoassays
One micron magnetic beads optimised for automated immunoassays
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
lab technology<br />
I mmunodiagnostics<br />
<strong>One</strong> <strong>micron</strong> <strong>magnetic</strong> <strong>beads</strong><br />
<strong>optimised</strong> <strong>for</strong> <strong>automated</strong><br />
<strong>immunoassays</strong><br />
by Jack Andreassen<br />
As Published in CLI April 2005<br />
The use of <strong>magnetic</strong> <strong>beads</strong> as the solid phase in <strong>automated</strong> <strong>immunoassays</strong> results<br />
in faster reactions and thus shorter assay times, as well as increased sensitivity.<br />
This article describes how the utilisation of a new one <strong>micron</strong> bead plat<strong>for</strong>m<br />
allows a larger surface area <strong>for</strong> protein immobilisation. Fewer <strong>beads</strong> can be used<br />
without compromising either the accuracy of results or the dynamic range.<br />
The improved dispersion properties of the new <strong>beads</strong>, combined with their low<br />
sedimentation rate, removes the need <strong>for</strong> mixing of reagents.<br />
The ever increasing pressures on clinical laboratories to become<br />
more productive and more cost-effective are <strong>for</strong>cing scientists to<br />
re-evaluate the way they carry out test procedures. Since labour<br />
accounts <strong>for</strong> approximately 65% of the total cost of producing<br />
test results, the most logical solution to the problem of cost control<br />
seems to be the automation of the most commonly per<strong>for</strong>med<br />
assays, such as <strong>immunoassays</strong>. However, any newly <strong>automated</strong><br />
test must generate results that match or are superior to<br />
those obtained with manually per<strong>for</strong>med assays. In addition, a<br />
much higher throughput should be attained with minimal<br />
requirements <strong>for</strong> specialised hands-on-time. To satisfy such challenging<br />
criteria, the design of the <strong>automated</strong> assay, and in particular<br />
the solid phase, is critical.<br />
The type of solid phase employed in <strong>automated</strong> <strong>immunoassays</strong> is<br />
a fundamental part of the assay design process and may ultimately<br />
determine the success of the assay. The solid phase must be easy<br />
to use and adaptable to assay development, should have high<br />
capacity, have excellent signal-to-noise ratios, and enable efficient<br />
and reproducible immobilisation of antibodies or other ligands.<br />
Minimal mixing requirements and rapid, effective washing of the<br />
solid phase are also essential in high throughput systems.<br />
The use of <strong>magnetic</strong> <strong>beads</strong> as the solid phase in <strong>automated</strong><br />
<strong>immunoassays</strong> is well established and gaining popularity. As more<br />
systems are developed and the assays become more complex, bead<br />
manufacturers are looking at ways to improve the properties of<br />
their <strong>beads</strong> to meet the growing needs of their customers. Dynal<br />
Biotech has developed a new one <strong>micron</strong> <strong>magnetic</strong> bead plat<strong>for</strong>m,<br />
Dyna<strong>beads</strong> My<strong>One</strong>, which has been <strong>optimised</strong> <strong>for</strong> use as the solid<br />
phase in <strong>automated</strong> <strong>immunoassays</strong>. Both carboxylic acid and<br />
tosylactivated <strong>for</strong>ms are available. The small, uni<strong>for</strong>m size of the<br />
<strong>beads</strong> provides a large surface area to which antibodies or other<br />
ligands can bind, while the carefully controlled iron content<br />
ensures that the <strong>beads</strong> are truly superpara<strong>magnetic</strong>. This means<br />
that there is no remanence (magnetism remaining) after removing<br />
the <strong>magnetic</strong> field, so preventing clumping in <strong>automated</strong> systems.<br />
The unique properties of the new <strong>beads</strong> have been carefully<br />
engineered to provide optimum results in <strong>automated</strong> <strong>immunoassays</strong>,<br />
described in more detail below.<br />
Bead size and size distribution<br />
To produce accurate and reproducible results in <strong>automated</strong><br />
<strong>immunoassays</strong>, all <strong>beads</strong> used as the solid phase must be identical.<br />
Coulter Counter measurements can be per<strong>for</strong>med to determine<br />
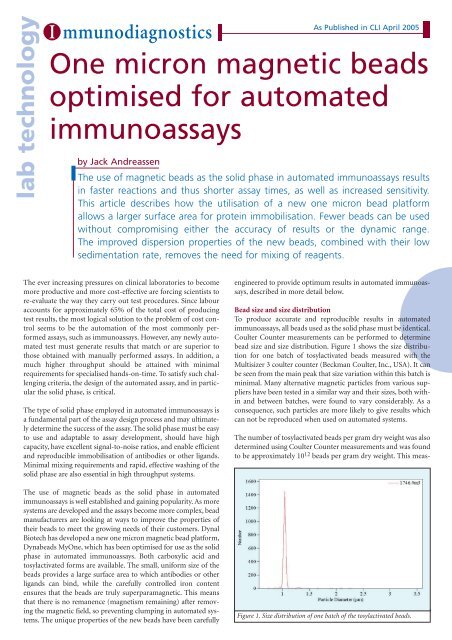
bead size and size distribution. Figure 1 shows the size distribution<br />
<strong>for</strong> one batch of tosylactivated <strong>beads</strong> measured with the<br />
Multisizer 3 coulter counter (Beckman Coulter, Inc., USA). It can<br />
be seen from the main peak that size variation within this batch is<br />
minimal. Many alternative <strong>magnetic</strong> particles from various suppliers<br />
have been tested in a similar way and their sizes, both within<br />
and between batches, were found to vary considerably. As a<br />
consequence, such particles are more likely to give results which<br />
can not be reproduced when used on <strong>automated</strong> systems.<br />
The number of tosylactivated <strong>beads</strong> per gram dry weight was also<br />
determined using Coulter Counter measurements and was found<br />
to be approximately 10 12 <strong>beads</strong> per gram dry weight. This meas-<br />
Figure 1. Size distribution of one batch of the tosylactivated <strong>beads</strong>.
I mmunodiagnostics<br />
As Published in CLI April 2005<br />
Figure 2. Hysteresis curve <strong>for</strong> the bead plat<strong>for</strong>m: magnetisation as a function<br />
of <strong>magnetic</strong> field strength. The magnetisation curves are overlapping<br />
both when increasing and decreasing the <strong>magnetic</strong> field, and the <strong>beads</strong><br />
show superpara<strong>magnetic</strong> behaviour.<br />
Figure 3. Sedimentation of 1.0 µm and 2.8 µm <strong>beads</strong> in aqueous solution<br />
measured as relative absorbance at 450 nm as a function of settling<br />
time (minutes).<br />
urement is dependent on bead diameter since the volume of the<br />
bead is proportional to the cube of the radius.<br />
Magnetic properties<br />
The <strong>magnetic</strong> properties of <strong>beads</strong> used as the solid phase in<br />
<strong>automated</strong> systems are clearly of great importance, both during<br />
the manufacture of the immunoassay and in its application. To<br />
ensure that the <strong>beads</strong> are collected efficiently on the magnet the<br />
iron oxide content must be high. However, total resuspension of<br />
the pellet during washing steps is equally important as rapid,<br />
effective washing steps reduce assay time and there<strong>for</strong>e increase<br />
overall throughput. To satisfy these requirements the <strong>beads</strong><br />
must be truly superpara<strong>magnetic</strong> and there should be no remanence<br />
after removing the <strong>magnetic</strong> field.<br />
The iron oxide content of Dyna<strong>beads</strong> My<strong>One</strong> <strong>beads</strong> is 37%. The<br />
<strong>magnetic</strong> material is evenly distributed throughout the <strong>beads</strong> as<br />
nanosized iron oxide crystals. This results in superpara<strong>magnetic</strong><br />
behaviour, as shown by the hysteresis curve in Figure 2, where<br />
the magnetisation is nearly identical with both increasing and<br />
decreasing <strong>magnetic</strong> fields. The <strong>beads</strong> show no remanence<br />
when the <strong>magnetic</strong> field is zero.<br />
Surface characteristics<br />
Hydrophobicity and the charge on the bead surface are important<br />
parameters when coating <strong>beads</strong> with antibodies or similar<br />
proteins generally needed in <strong>immunoassays</strong>. The initial driving<br />
Table 1. Relative contact angle and isoelectric point measured with Zeta potential.<br />
<strong>for</strong>ce when immobilising antibodies on hydrophobic <strong>beads</strong>,<br />
such as the tosylactivated <strong>beads</strong>, is hydrophobic adsorption to<br />
the bead surface. The chemical covalent bonds between the<br />
tosylactivated groups on the bead surface and the protein are<br />
only created after this initial contact. For hydrophilic bead surfaces<br />
the initial contact between the antibody and the surface<br />
of the bead is electrostatic or via random interactions, or it<br />
may occur following activation of functional groups on the<br />
bead surface.<br />
The degree of hydrophobicity of the tosylactivated <strong>beads</strong> as well<br />
as the carboxylic acid version of the <strong>beads</strong> was determined by<br />
measuring the contact angle of water droplets deposited on a<br />
layer of dry <strong>beads</strong> using a Fibro Dat 1120 instrument (Thwing-<br />
Albert Instrument Company, USA). The higher the contact<br />
angle observed, the more hydrophobic the bead surface.<br />
The isoelectric point <strong>for</strong> each of the bead types was also determined<br />
using the Zetasizer 2000-3000 HS (Malvern<br />
Instruments, UK) to measure the Zeta potential. These measurements<br />
were per<strong>for</strong>med on uncoated <strong>beads</strong> as well as on<br />
<strong>beads</strong> coated with antibody or streptavidin, to assess whether<br />
the surface properties of the <strong>beads</strong> are affected by the immobilised<br />
protein. The results are shown in Table 1.<br />
The hydrophobicity of the tosylactivated <strong>beads</strong> is very comparable,<br />
whereas the carboxylic acid <strong>beads</strong> are, in contrast, very<br />
hydrophilic due to their high negative<br />
net charge at neutral pH. Zeta potential<br />
measurements were per<strong>for</strong>med on three<br />
different types of <strong>beads</strong> (two sizes of<br />
tosylactivated <strong>beads</strong> and carboxylic acid<br />
<strong>beads</strong>) after coating with monoclonal<br />
mouse IgG1 antibody and streptavidin<br />
[Table 1]. These results show that the<br />
isoelectric point of the <strong>beads</strong> is principally<br />
determined by the nature of the<br />
immobilised protein. For example, after<br />
coating the originally negatively charged<br />
carboxylic acid <strong>beads</strong> with antibody,<br />
their isoelectric point is similar to the<br />
more neutral tosylactivated <strong>beads</strong> coated<br />
with the same antibody.
I mmunodiagnostics<br />
As Published in CLI April 2005<br />
Understanding the surface characteristics of the different types<br />
of <strong>beads</strong> is important in immunoassay development in order to<br />
establish the optimal binding kinetics <strong>for</strong> both the ligand binding<br />
to the bead surface, as well as <strong>for</strong> the target protein binding<br />
to the immobilised ligand. Electrostatic interactions (repulsion<br />
<strong>for</strong>ces) may lower the binding efficiency, particularly if the surface<br />
of the <strong>beads</strong> is negatively charged.<br />
Antibody immobilisation<br />
The immobilisation efficiency of the coating of <strong>magnetic</strong> <strong>beads</strong><br />
with proteins such as antibodies or streptavidin, is very important<br />
since it affects the number of <strong>beads</strong> required in the solid phase as<br />
well as the dynamic range that can be achieved by the assay. The<br />
rate of immobilisation depends on the properties of the protein,<br />
such as its size and isoelectric point, as well as the relative concentrations<br />
of the protein and <strong>magnetic</strong> <strong>beads</strong>. The nature of the<br />
buffer used during the immobilisation is also important. To measure<br />
the efficiency of antibody immobilisation to the tosylactivated<br />
<strong>beads</strong>, I 125 -labelled antibody was added to the <strong>beads</strong>. It was found<br />
that, depending on the antibody type, 60-95% of the antibody was<br />
bound to the <strong>beads</strong> following incubation. The degree of covalent<br />
binding was then measured using SDS to remove physically<br />
adsorbed protein. It was found that 95-100% of the antibodies<br />
bound to the tosylactivated <strong>beads</strong> were linked covalently, thus<br />
reducing the risk of protein leakage from the <strong>beads</strong> during storage.<br />
Sedimentation<br />
In <strong>automated</strong> <strong>immunoassays</strong> the opportunities <strong>for</strong> mixing during<br />
incubation and washing steps may be limited. It is there<strong>for</strong>e<br />
important <strong>for</strong> <strong>beads</strong> to have a slow sedimentation rate to prevent<br />
them from settling at the bottom of the cuvette. Both the size and<br />
density of the <strong>beads</strong> determine the rate of sedimentation, which<br />
can be measured using a spectrophotometer as the <strong>beads</strong> are<br />
allowed to settle over time. As the <strong>beads</strong> settle the solution becomes<br />
less dense optically, and consequently the absorbance reading at<br />
450 nm decreases. The sedimentation rate <strong>for</strong> 2.8 µm streptavidin<br />
<strong>beads</strong> and 1.0 µm streptavidin <strong>beads</strong> were compared over time and<br />
the results are shown in Figure 3. Both the<br />
2.8 µm and the 1.0 µm <strong>beads</strong> remained in<br />
suspension <strong>for</strong> the first 30 minutes. While<br />
the 2.8 µm <strong>beads</strong> almost completely settled<br />
after 100 minutes, the 1.0 µm <strong>beads</strong> were<br />
still in solution after 3 hours. It can be concluded,<br />
there<strong>for</strong>e, that using the 1.0 µm<br />
streptavidin <strong>beads</strong> in <strong>automated</strong><br />
<strong>immunoassays</strong> eliminates the need <strong>for</strong><br />
mixing during the incubation steps.<br />
Figure 4. Bead titration of different amounts of 1.0 µm <strong>beads</strong> in a D-<br />
dimer immunoassay compared with 2.8 µm <strong>beads</strong>..<br />
To determine whether this is the case, 2.8 µm tosylactivated <strong>beads</strong><br />
and 1µm tosylactivated <strong>beads</strong> were coated with antibody against<br />
D-dimer in a model system and used in a sandwich immunoassay<br />
<strong>for</strong> D-dimer on an <strong>automated</strong> random access bench top<br />
analyser. As a reference, the required weight of the 2.8 µm tosylactivated<br />
<strong>beads</strong> was taken to be 100%. Preparations containing<br />
30, 40 and 50% weight of the 1µm tosylactivated <strong>beads</strong> were then<br />
used in the assay and the results compared to those obtained with<br />
the 2.8 µm <strong>beads</strong> Figure 4. The results indicate that the increased<br />
surface area of the 1.0 µm <strong>beads</strong> reduced the weight of <strong>beads</strong><br />
required in the assay by 60%. This may be used to increase the<br />
dynamic range of the assay.<br />
This conclusion that a reduced weight of 1.0 µm <strong>beads</strong> is needed<br />
compared to 2.8 µm <strong>beads</strong> is also valid with other assays. A number<br />
of further experiments were per<strong>for</strong>med. Both bead sizes were<br />
coated with capture antibodies <strong>for</strong> D-dimer and myoglobulin <strong>for</strong><br />
use in a sandwich immunoassay on a <strong>automated</strong> random access<br />
bench top analyser. Two preparations of different sizes of streptavidin-coated<br />
<strong>beads</strong> were also used in a sandwich immunoassay<br />
<strong>for</strong> intact parathyroid hormone (PTH) utilising a biotinylated<br />
Titration of the <strong>beads</strong> in <strong>immunoassays</strong><br />
The number of <strong>beads</strong> required in the solid<br />
phase of an immunoassay may be determined<br />
by the need <strong>for</strong> a wide dynamic<br />
range. In addition to parameters such as<br />
immobilisation efficiency, the size of the<br />
<strong>beads</strong> is also important since smaller <strong>beads</strong><br />
have a larger surface area available <strong>for</strong><br />
immobilisation. As the geometrical surface<br />
area per weight <strong>for</strong> 1.0 µm <strong>beads</strong> is approximately<br />
2.5 times higher than 2.8 µm<br />
<strong>beads</strong>, theoretically fewer 1.0 µm <strong>beads</strong><br />
should be required to achieve the same<br />
accuracy in an assay.<br />
Figure 5. Standard curve measurement <strong>for</strong> D-dimer immunoassay with 2.8 µm <strong>beads</strong> (100 %<br />
weight), and 1µm <strong>beads</strong> (40 % weight).
I mmunodiagnostics<br />
As Published in CLI April 2005<br />
Figure 6. Standard curve measurement <strong>for</strong> Myoglobulin immunoassay<br />
with 2.8 µm <strong>beads</strong> (100 % weight), and 1µm <strong>beads</strong> (40 % weight).<br />
Figure 7. Standard curve measurement <strong>for</strong> intact PTH immunoassay<br />
with 2.8 µm <strong>beads</strong> (100 % weight), and 1µm <strong>beads</strong> (40 % weight).<br />
Figure 8. Response units in a sandwich PTH assay employing streptavidin coated <strong>beads</strong> and a<br />
biotinylated capture antibody as a function of the measured free biotin binding capacity of the streptavidin<br />
coated <strong>beads</strong>. The validation batches (red) compare very well with the process qualification<br />
batch (purple). Experimental batches with different biotin binding capacities are depicted in blue.<br />
capture antibody as a model system. The results, shown in Figures<br />
5, 6 and 7, confirm that the amount of 1µm <strong>beads</strong> required in all<br />
assays is just 40% of the weight required of 2.8 µm <strong>beads</strong>.<br />
Reproducibility<br />
When manufacturing assay components such as <strong>magnetic</strong> <strong>beads</strong><br />
coated with antibodies or similar proteins, reproducible results<br />
are essential. All batches of the 1µm <strong>beads</strong> are validated at the production<br />
scale. Validation batches are then further analysed using<br />
standard QC methods to ensure that the physical and chemical<br />
properties of the <strong>beads</strong> in each batch are identical. Three different<br />
batches of the tosylactivated <strong>beads</strong> were coated with streptavidin<br />
and tested in an immunoassay <strong>for</strong> PTH utilising a biotinylated<br />
capture antibody. Experimental batches of <strong>beads</strong> with lower<br />
biotin binding capacities were also included to determine the<br />
threshold biotin binding capacity required to achieve reproducible<br />
results in the PTH assay. The results, shown in Figure 8,<br />
demonstrate the excellent reproducibility of the 1.0 µm bead<br />
manufacturing processes, since the validation batches (shown in<br />
red) compare very well with the process qualification batch<br />
(shown in purple). The low-binding<br />
experimental batches are shown in blue.<br />
Similarly, the validation batches were<br />
coated directly with PTH antibody and<br />
tested in a standard sandwich<br />
immunoassay. All four batches of tosylactivated<br />
<strong>beads</strong> behaved identically in the<br />
PTH assay, demonstrating the excellent<br />
reproducibility of the antibody immobilisation<br />
process used to coat the tosylactivated<br />
<strong>beads</strong>.<br />
Conclusion<br />
Dyna<strong>beads</strong> My<strong>One</strong> <strong>beads</strong> have been carefully<br />
engineered to create an optimum<br />
<strong>magnetic</strong> solid phase <strong>for</strong> <strong>automated</strong><br />
<strong>immunoassays</strong>. Their small, uni<strong>for</strong>m size<br />
provides a large surface area <strong>for</strong> consistent<br />
protein immobilisation and enables<br />
60% less weight of <strong>beads</strong> to be used in<br />
assays without compromising the accuracy<br />
or the dynamic range. Good dispersion<br />
properties combined with a slow sedimentation<br />
rate remove the need <strong>for</strong> stirring<br />
during incubation and washing steps, while the high iron<br />
oxide content ensures excellent separation properties with no<br />
remanence. The <strong>beads</strong> can be used to improve the dynamic range<br />
and signal-to-noise ratio of existing <strong>immunoassays</strong> with greatly<br />
reduced need <strong>for</strong> assay optimisation.<br />
The author<br />
Jack Andreassen, M.Sc.,<br />
Senior International Product Manager,<br />
IVD / OEM Support<br />
Dynal Biotech,<br />
Ullernchausseen 52N-0309,<br />
Oslo,<br />
Norway<br />
The work was carried out in collaboration with<br />
Future Diagnostics, bv.