You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Lasers in Medical Manufacturing<br />
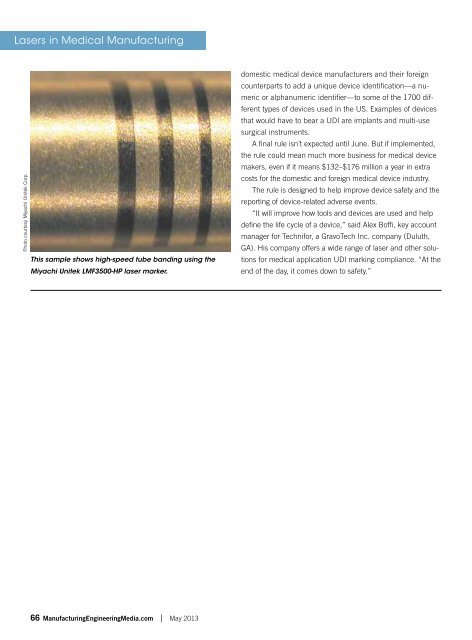
Photo courtesy Miyachi Unitek Corp.<br />
This sample shows high-speed tube banding using the<br />
Miyachi Unitek LMF3500-HP laser marker.<br />
domestic medical device manufacturers and their <strong>for</strong>eign<br />
counterparts to add a unique device identification—a numeric<br />
or alphanumeric identifier—to some of the 1700 different<br />
types of devices used in the US. Examples of devices<br />
that would have to bear a UDI are implants and multi-use<br />
surgical instruments.<br />
A final rule isn’t expected until June. But if implemented,<br />
the rule could mean much more business <strong>for</strong> medical device<br />
makers, even if it means $132–$176 million a year in extra<br />
costs <strong>for</strong> the domestic and <strong>for</strong>eign medical device industry.<br />
The rule is designed to help improve device safety and the<br />
reporting of device-related adverse events.<br />
“It will improve how tools and devices are used and help<br />
define the life cycle of a device,” said Alex Boffi, key account<br />
manager <strong>for</strong> Techni<strong>for</strong>, a GravoTech Inc. company (Duluth,<br />
GA). His company offers a wide range of laser and other solutions<br />
<strong>for</strong> medical application UDI marking compliance. “At the<br />
end of the day, it comes down to safety.”<br />
66 ManufacturingEngineeringMedia.com | May 2013