Squamous Cell Carcinoma Antigen: more sensitive second ...
Squamous Cell Carcinoma Antigen: more sensitive second ...
Squamous Cell Carcinoma Antigen: more sensitive second ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
C ancer biomarkers<br />
as published in CLI December 2004<br />
<strong>Squamous</strong> <strong>Cell</strong> <strong>Carcinoma</strong> <strong>Antigen</strong>:<br />
<strong>more</strong> <strong>sensitive</strong> <strong>second</strong> generation assays<br />
By Dr. R. Einarsson<br />
<strong>Squamous</strong> cell carcinoma antigen (SCCA) is a serological marker for squamous cell carcinomas<br />
(SCC) of the uterine cervix, lung, head and neck and oesophagus. The antigen<br />
is a group of cytoplasmic proteins found in normal squamous epithelia. The measurement<br />
of the serum levels of SCCA in patients with cancer of the cervix of the uterus has<br />
been reported to be useful for distinguishing between patients with and without a high<br />
risk of developing lymph node metastases. In addition, SCCA has been shown to correlate<br />
with the clinical course of the disease following treatment and to predict relapse<br />
[1-4]. Deep stromal infiltration and positive lymph nodes significantly were associated<br />
with increased SCCA level in many patients. However, about 30% to 50% of patients<br />
with cervical carcinoma have been shown to have normal SCCA concentration. In particular,<br />
<strong>more</strong> than 50% of patients in clinical FIGO stage I and II had normal SCCA levels.<br />
Thus, the determination of SCCA alone is not suitable for clinical purposes. For this<br />
reason several attempts have been made to try to overcome the limitation of SCCA on<br />
its own by the concomitant use of additional tumour markers.<br />
SCCA was first isolated by conventional protein purification methods from a cervical<br />
squamous cell carcinoma. Biochemical characterisation of the original protein fraction<br />
(TA-4), showed that it comprised a group of proteins with a molecular weight of<br />
approximately 45 kDa [1]. This protein fraction was then used to raise polyclonal antibodies<br />
for further characterisation and for the development of assays for serological<br />
studies [1]. The TA-4 fraction could be further separated in two subfractions by isoelectric<br />
focusing (acidic fraction pI6.25). Each of these subfractions<br />
were shown to consist of at least ten different protein components [1].<br />
Current assays of SCCA<br />
The clinical utility of serum SCCA assays has been best documented when they were<br />
being applied to the prognosis, monitoring therapy and follow-up of cervical cancer<br />
patients. It has been suggested that in the tumour there might be differences in the<br />
expression of the acidic and neutral protein fractions. Studies supported the hypothesis<br />
that the acidic SCCA fraction was <strong>more</strong> associated with cancer than the neutral fraction.<br />
Most clinical data on the utility of SCCA assays have been generated in studies<br />
using radioimmunoassay or fluorescence polarisation immunoassay in Abbott’s IMx<br />
SCC assay. Both SCCA1 and SCCA2 have been measured in these systems [1]. Serum<br />
SCC antigen levels correlate with clinical stage and treatment outcome in various carcinomas.<br />
High pre-treatment levels indicate extensive disease and poor prognosis for<br />
patients with cervical cancer of the squamous cell carcinoma histotype, but not for the<br />
adenocarcinomas. A good correlation has been reported between serum SCC antigen<br />
levels and the extent of disease [3]. Most SCCA data have been reported for cervical cancer<br />
patients, but due to low sensitivity and specificity SCCA has limited clinical utility in<br />
early-stage disease. Similar results have also been reported for patients with lung cancer<br />
of histological type squamous cell carcinoma and for patients with squamous cell carcinoma<br />
of the head and neck. New methods have been developed to measure serum<br />
SCCA1 or SCCA2 separately and by measuring recombinant SCCA preparations.<br />
However it has been shown that recombinant SCCA2 is underestimated using the IMx<br />
SCC assay [6]. In serum samples from cervical cancer patients, the SCC antigen consists<br />
mainly of the SCCA2 isomer and this might be one of the reason for the limited sensitivity<br />
of the available SCCA assays.<br />
Molecular diagnostics<br />
Molecular cloning has demonstrated that SCCA is produced by two almost identical<br />
genes named SCCA1 and SCCA2 [7]. The two SCC genes are tandemly arranged<br />
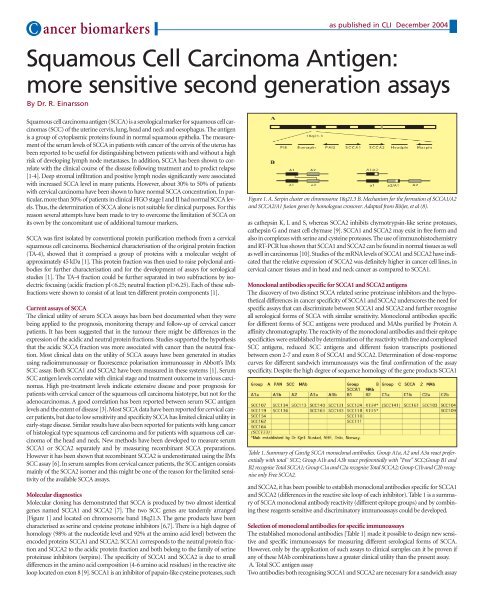
[Figure 1] and located on chromosome band 18q21.3. The gene products have been<br />
characterised as serine and cysteine protease inhibitors [6,7]. There is a high degree of<br />
homology (98% at the nucleotide level and 92% at the amino acid level) between the<br />
encoded proteins SCCA1 and SCCA2. SCCA1 corresponds to the neutral protein fraction<br />
and SCCA2 to the acidic protein fraction and both belong to the family of serine<br />
proteinase inhibitors (serpins). The specificity of SCCA1 and SCCA2 is due to small<br />
differences in the amino acid composition (4-6 amino acid residues) in the reactive site<br />
loop located on exon 8 [9]. SCCA1 is an inhibitor of papain-like cysteine proteases, such<br />
Figure 1. A. Serpin cluster on chromosome 18q21.3 B. Mechanism for the formation of SCCA1/A2<br />
and SCCA2/A1 fusion genes by homologous crossover. Adapted from Röijer, et al (8).<br />
as cathepsin K, L and S, whereas SCCA2 inhibits chymotrypsin-like serine proteases,<br />
cathepsin G and mast cell chymase [9]. SCCA1 and SCCA2 may exist in free form and<br />
also in complexes with serine and cysteine proteases. The use of immunohistochemistry<br />
and RT-PCR has shown that SCCA1 and SCCA2 can be found in normal tissues as well<br />
as well in carcinomas [10]. Studies of the mRNA levels of SCCA1 and SCCA2 have indicated<br />
that the relative expression of SCCA2 was definitely higher in cancer cell lines, in<br />
cervical cancer tissues and in head and neck cancer as compared to SCCA1.<br />
Monoclonal antibodies specific for SCCA1 and SCCA2 antigens<br />
The discovery of two distinct SCCA related serine proteinase inhibitors and the hypothetical<br />
differences in cancer specificity of SCCA1 and SCCA2 underscores the need for<br />
specific assays that can discriminate between SCCA1 and SCCA2 and further recognise<br />
all serological forms of SCCA with similar sensitivity. Monoclonal antibodies specific<br />
for different forms of SCC antigens were produced and MAbs purified by Protein A<br />
affinity chromatography. The reactivity of the monoclonal antibodies and their epitope<br />
specificities were established by determination of the reactivity with free and complexed<br />
SCC antigens, reduced SCC antigens and different fusion transcripts positioned<br />
between exon 2-7 and exon 8 of SCCA1 and SCCA2. Determination of dose-response<br />
curves for different sandwich immunoassays was the final confirmation of the assay<br />
specificity. Despite the high degree of sequence homology of the gene products SCCA1<br />
Table 1. Summary of CanAg SCCA monoclonal antibodies. Group A1a, A2 and A3a react preferentially<br />
with total¨ SCC; Group A1b and A3b react preferentially with "Free" SCC;Group B1 and<br />
B2 recognise Total SCCA1; Group C1a and C2a recognise Total SCCA2; Group C1b and C2b recognise<br />
only Free SCCA2.<br />
and SCCA2, it has been possible to establish monoclonal antibodies specific for SCCA1<br />
and SCCA2 (differences in the reactive site loop of each inhibitor). Table 1 is a summary<br />
of SCCA monoclonal antibody reactivity (different epitope groups) and by combining<br />
these reagents <strong>sensitive</strong> and discriminatory immunoassays could be developed.<br />
Selection of monoclonal antibodies for specific immunoassays<br />
The established monoclonal antibodies [Table 1] made it possible to design new <strong>sensitive</strong><br />
and specific immunoassays for measuring different serological forms of SCCA.<br />
However, only by the application of such assays to clinical samples can it be proven if<br />
any of these MAb combinations have a greater clinical utility than the present assay.<br />
A. Total SCC antigen assay<br />
Two antibodies both recognising SCCA1 and SCCA2 are necessary for a sandwich assay
C ancer biomarkers<br />
as published in CLI December 2004<br />
measuring total or Pan SCC. Optimal reactivity in the ELISA assay for measuring total<br />
SCC was obtained with the antibody pair SCC140 (catcher antibody) and SCC107<br />
(detector antibody).<br />
B. Total SCCA1 assay<br />
Combination of monoclonal antibodies from Group B and monoclonal antibodies<br />
from Group A permitted the design of specific immunoassays for SCCA1 antigen. A<br />
monoclonal antibody characterised by high sensitivity for SCCA1 antigen and low<br />
cross-reactivity with SCCA2 antigen was selected. Optimal reactivity in the immunoassay<br />
for measuring total SCCA1 was obtained with the antibody pair SCC111 (catcher<br />
antibody) and SCC107 (detector antibody).<br />
9 Luke C et al. Biochemistry 2000; 39: 7081.<br />
10. Cataltepe S et al. J Histochem Cytochem 2000; 48: 113.<br />
The author<br />
R. Einarsson, Ph. D.,<br />
Biotech Division,<br />
CanAg Diagnostics AB<br />
SE 41455 Gothenburg<br />
Sweden<br />
Fax + 46 31 85 70 40<br />
E mail roland.einarsson@canag.se<br />
C. Total SCCA2 assay<br />
Different antibodies from Group A and Group C [Table 1] can be selected for measuring<br />
total SCCA2 antigen. Three monoclonal antibodies recognising free SCCA2 and<br />
two monoclonal antibodies recognising total SCCA2 antigen were established. Optimal<br />
reactivity for measuring total SCCA2 antigen (Free SCCA2 and SCCA2 in complex<br />
with proteases) was obtained with the antibody pair SCC103 (catcher antibody) and<br />
SCC107 (detector antibody).<br />
D. Free SCCA2 assay<br />
Three antibodies from Group C (SCC104, SCC 109 and SCC161) were found to be specific<br />
for free SCCA2 antigen. All antibodies demonstrated low cross-reactivity with<br />
SCCA1 antigen. The preferred configuration for measuring free SCCA2 antigen was<br />
based upon the pair SCC 104 (catcher antibody) and SCC107 (detector antibody).<br />
Clinical evidence for the role of CanAg SCCA specific assays<br />
SCCA is the most useful serological marker for cervical cancer and has demonstrated<br />
its clinical value for therapy monitoring and for establishing prognosis. However, this<br />
biomarker is not <strong>sensitive</strong> enough for the early diagnosis of cervical cancer. Using the<br />
newly developed ELISA assay for measuring the different serological forms of SCCA, it<br />
was shown that SCCA2 was the dominating serological form of SCCA in healthy subjects<br />
and in most cervical cancer patients (FIGO stage II-IV) [5]. However, both SCCA1<br />
and SCCA2 followed the clinical course of the disease, and could be used for monitoring<br />
treatment outcome in cervical cancer patients and also to predict progressive disease.<br />
In most patients SCCA2 antigen showed the most pronounced elevation of the<br />
SCCA antigens in clinically confirmed progressive disease. In some patients however the<br />
SCCA1 antigen was the earliest predictor of progressive disease. Optimal clinical sensitivity<br />
and specificity would be obtained using an assay measuring all serological forms<br />
of SCCA.<br />
Future Prospects for SCCA1 and SCCA2<br />
The SCC antigen is an established biomarker for patient management in cervical cancer,<br />
lung cancer and head and neck cancer patients. Levels of the antigen can provide<br />
information on prognosis, treatment outcome and the prediction of recurrence.<br />
However there is a great need for cancer markers that will further improve these aspects.<br />
In this respect, the <strong>second</strong> generation SCCA assays such as those described above may<br />
be useful for the early detection of disease and prediction of progressive disease. Studies<br />
with SCCA1 and SCCA2 have shown that for optimal clinical sensitivity, an assay recognising<br />
both SCCA1 and SCCA2 with similar sensitivity is to be preferred. The specific<br />
determination of SCCA1 and SCCA2 antigen levels may provide additional clinical<br />
information compared to the determination of Total SCC. In conclusion, it might be<br />
possible to increase the sensitivity and specificity of the measurement of SCCA in early<br />
cancer diseases such cervical, lung and head and neck cancer by applying the new <strong>second</strong><br />
generation monoclonal antibodies. The new antibodies will also be useful to characterise<br />
the native proteases associated with SCCA1 and SCCA2 in squamous cell carcinoma<br />
and normal tissues.<br />
References<br />
1. Kato H in Serological Cancer Markers (S. Sell ed.), Totowa, Humana Press, pp. 437-<br />
451 (1992).<br />
2. Snyderman CH et al. Arch Otalaryngol Head Neck Surg 1995; 121: 1294.<br />
3. de Bruijn HWA et al. Tumour Biol 1998; 19: 505.<br />
4.Takeda A et al. Biol Chem 2002; 383: 1231.<br />
5. de Bruijn HWA et al. Tumour Biol 2003; 24: 83.<br />
6.Cataltepe S et al. Clin Chim Acta 2000; 295: 107.<br />
7.Suminami Y et al. Tumour Biol 1998; 19: 488.<br />
8. Röijer E et al. Tumour Biol 2003; 24: 46.