ë¸ë¡ì (PDF)
ë¸ë¡ì (PDF)
ë¸ë¡ì (PDF)
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
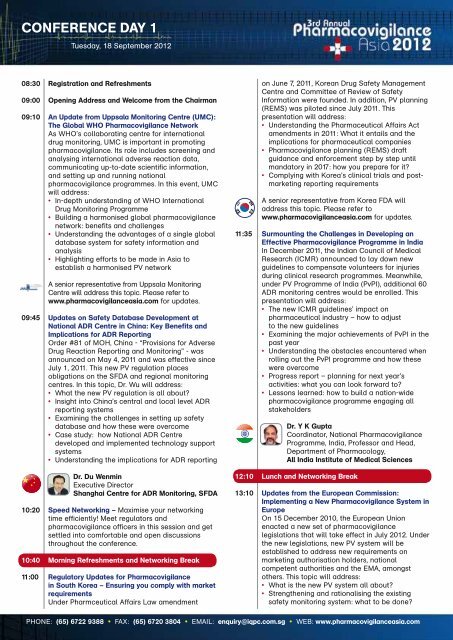
CONFERENCE DAY 1<br />
Tuesday, 18 September 2012<br />
08:30 Registration and Refreshments<br />
09:00 Opening Address and Welcome from the Chairman<br />
09:10 An Update from Uppsala Monitoring Centre (UMC):<br />
The Global WHO Pharmacovigilance Network<br />
As WHO’s collaborating centre for international<br />
drug monitoring, UMC is important in promoting<br />
pharmacovigilance. Its role includes screening and<br />
analysing international adverse reaction data,<br />
communicating up-to-date scientific information,<br />
and setting up and running national<br />
pharmacovigilance programmes. In this event, UMC<br />
will address:<br />
• In-depth understanding of WHO International<br />
Drug Monitoring Programme<br />
• Building a harmonised global pharmacovigilance<br />
network: benefits and challenges<br />
• Understanding the advantages of a single global<br />
database system for safety information and<br />
analysis<br />
• Highlighting efforts to be made in Asia to<br />
establish a harmonised PV network<br />
A senior representative from Uppsala Monitoring<br />
Centre will address this topic. Please refer to<br />
www.pharmacovigilanceasia.com for updates.<br />
09:45 Updates on Safety Database Development at<br />
National ADR Centre in China: Key Benefits and<br />
Implications for ADR Reporting<br />
Order #81 of MOH, China - “Provisions for Adverse<br />
Drug Reaction Reporting and Monitoring” - was<br />
announced on May 4, 2011 and was effective since<br />
July 1, 2011. This new PV regulation places<br />
obligations on the SFDA and regional monitoring<br />
centres. In this topic, Dr. Wu will address:<br />
• What the new PV regulation is all about<br />
• Insight into China’s central and local level ADR<br />
reporting systems<br />
• Examining the challenges in setting up safety<br />
database and how these were overcome<br />
• Case study: how National ADR Centre<br />
developed and implemented technology support<br />
systems<br />
• Understanding the implications for ADR reporting<br />
Dr. Du Wenmin<br />
Executive Director<br />
Shanghai Centre for ADR Monitoring, SFDA<br />
10:20 Speed Networking – Maximise your networking<br />
time efficiently! Meet regulators and<br />
pharmacovigilance officers in this session and get<br />
settled into comfortable and open discussions<br />
throughout the conference.<br />
10:40 Morning Refreshments and Networking Break<br />
11:00 Regulatory Updates for Pharmacovigilance<br />
in South Korea – Ensuring you comply with market<br />
requirements<br />
Under Pharmceutical Affairs Law amendment<br />
on June 7, 2011, Korean Drug Safety Management<br />
Centre and Committee of Review of Safety<br />
Information were founded. In addition, PV planning<br />
(REMS) was piloted since July 2011. This<br />
presentation will address:<br />
• Understanding the Pharmaceutical Affairs Act<br />
amendments in 2011: What it entails and the<br />
implications for pharmaceutical companies<br />
• Pharmacovigilance planning (REMS) draft<br />
guidance and enforcement step by step until<br />
mandatory in 2017: how you prepare for it<br />
• Complying with Korea’s clinical trials and postmarketing<br />
reporting requirements<br />
A senior representative from Korea FDA will<br />
address this topic. Please refer to<br />
www.pharmacovigilanceasia.com for updates.<br />
11:35 Surmounting the Challenges in Developing an<br />
Effective Pharmacovigilance Programme in India<br />
In December 2011, the Indian Council of Medical<br />
Research (ICMR) announced to lay down new<br />
guidelines to compensate volunteers for injuries<br />
during clinical research programmes. Meanwhile,<br />
under PV Programme of India (PvPI), additional 60<br />
ADR monitoring centres would be enrolled. This<br />
presentation will address:<br />
• The new ICMR guidelines’ impact on<br />
pharmaceutical industry – how to adjust<br />
to the new guidelines<br />
• Examining the major achievements of PvPI in the<br />
past year<br />
• Understanding the obstacles encountered when<br />
rolling out the PvPI programme and how these<br />
were overcome<br />
• Progress report – planning for next year’s<br />
activities: what you can look forward to<br />
• Lessons learned: how to build a nation-wide<br />
pharmacovigilance programme engaging all<br />
stakeholders<br />
Dr. Y K Gupta<br />
Coordinator, National Pharmacovigilance<br />
Programme, India, Professor and Head,<br />
Department of Pharmacology,<br />
All India Institute of Medical Sciences<br />
12:10 Lunch and Networking Break<br />
13:10 Updates from the European Commission:<br />
Implementing a New Pharmacovigilance System in<br />
Europe<br />
On 15 December 2010, the European Union<br />
enacted a new set of pharmacovigilance<br />
legislations that will take effect in July 2012. Under<br />
the new legislations, new PV system will be<br />
established to address new requirements on<br />
marketing authorisation holders, national<br />
competent authorities and the EMA, amongst<br />
others. This topic will address:<br />
• What is the new PV system all about<br />
• Strengthening and rationalising the existing<br />
safety monitoring system: what to be done<br />
PHONE: (65) 6722 9388 • FAX: (65) 6720 3804 • EMAIL: enquiry@iqpc.com.sg • WEB: www.pharmacovigilanceasia.com