ë¸ë¡ì (PDF)
ë¸ë¡ì (PDF)
ë¸ë¡ì (PDF)
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
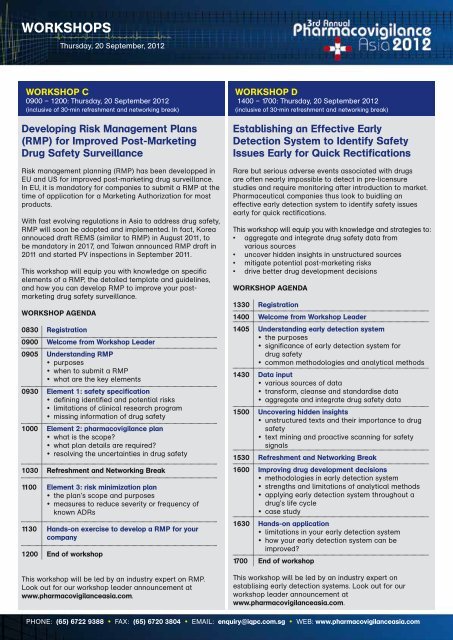
WORKSHOPS<br />
Thursday, 20 September, 2012<br />
WORKSHOP C<br />
0900 – 1200: Thursday, 20 September 2012<br />
(inclusive of 30-min refreshment and networking break)<br />
Developing Risk Management Plans<br />
(RMP) for Improved Post-Marketing<br />
Drug Safety Surveillance<br />
Risk management planning (RMP) has been developped in<br />
EU and US for improved post-marketing drug surveillance.<br />
In EU, it is mandatory for companies to submit a RMP at the<br />
time of application for a Marketing Authorization for most<br />
products.<br />
With fast evolving regulations in Asia to address drug safety,<br />
RMP will soon be adopted and implemented. In fact, Korea<br />
annouced draft REMS (similar to RMP) in August 2011, to<br />
be mandatory in 201 7, and Taiwan announced RMP draft in<br />
2011 and started PV inspections in September 2011.<br />
This workshop will equip you with knowledge on specific<br />
elements of a RMP, the detailed template and guidelines,<br />
and how you can develop RMP to improve your postmarketing<br />
drug safety surveillance.<br />
WORKSHOP AGENDA<br />
0830 Registration<br />
0900 Welcome from Workshop Leader<br />
0905 Understanding RMP<br />
• purposes<br />
• when to submit a RMP<br />
• what are the key elements<br />
0930 Element 1: safety specification<br />
• defining identified and potential risks<br />
• limitations of clinical research program<br />
• missing information of drug safety<br />
1000 Element 2: pharmacovigilance plan<br />
• what is the scope<br />
• what plan details are required<br />
• resolving the uncertainties in drug safety<br />
1030 Refreshment and Networking Break<br />
1100 Element 3: risk minimization plan<br />
• the plan’s scope and purposes<br />
• measures to reduce severity or frequency of<br />
known ADRs<br />
1130 Hands-on exercise to develop a RMP for your<br />
company<br />
1200 End of workshop<br />
This workshop will be led by an industry expert on RMP.<br />
Look out for our workshop leader announcement at<br />
www.pharmacovigilanceasia.com.<br />
WORKSHOP D<br />
1400 – 1700: Thursday, 20 September 2012<br />
(inclusive of 30-min refreshment and networking break)<br />
Establishing an Effective Early<br />
Detection System to Identify Safety<br />
Issues Early for Quick Rectifications<br />
Rare but serious adverse events associated with drugs<br />
are often nearly impossible to detect in pre-licensure<br />
studies and require monitoring after introduction to market.<br />
Pharmaceutical companies thus look to buidling an<br />
effective early detection system to identify safety issues<br />
early for quick rectifications.<br />
This workshop will equip you with knowledge and strategies to:<br />
• aggregate and integrate drug safety data from<br />
various sources<br />
• uncover hidden insights in unstructured sources<br />
• mitigate potential post-marketing risks<br />
• drive better drug development decisions<br />
WORKSHOP AGENDA<br />
1330 Registration<br />
1400 Welcome from Workshop Leader<br />
1405 Understanding early detection system<br />
• the purposes<br />
• significance of early detection system for<br />
drug safety<br />
• common methodologies and analytical methods<br />
1430 Data input<br />
• various sources of data<br />
• transform, cleanse and standardise data<br />
• aggregate and integrate drug safety data<br />
1500 Uncovering hidden insights<br />
• unstructured texts and their importance to drug<br />
safety<br />
• text mining and proactive scanning for safety<br />
signals<br />
1530 Refreshment and Networking Break<br />
1600 Improving drug development decisions<br />
• methodologies in early detection system<br />
• strengths and limitations of analytical methods<br />
• applying early detection system throughout a<br />
drug’s life cycle<br />
• case study<br />
1630 Hands-on application<br />
• limitations in your early detection system<br />
• how your early detection system can be<br />
improved<br />
1700 End of workshop<br />
This workshop will be led by an industry expert on<br />
establising early detection systems. Look out for our<br />
workshop leader announcement at<br />
www.pharmacovigilanceasia.com.<br />
PHONE: (65) 6722 9388 • FAX: (65) 6720 3804 • EMAIL: enquiry@iqpc.com.sg • WEB: www.pharmacovigilanceasia.com