Haematologica 2000;85:supplement to no. 10 - Supplements ...
Haematologica 2000;85:supplement to no. 10 - Supplements ...
Haematologica 2000;85:supplement to no. 10 - Supplements ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Immune Tolerance and the Treatment of Hemophilacs with an Inhibi<strong>to</strong>r 65<br />
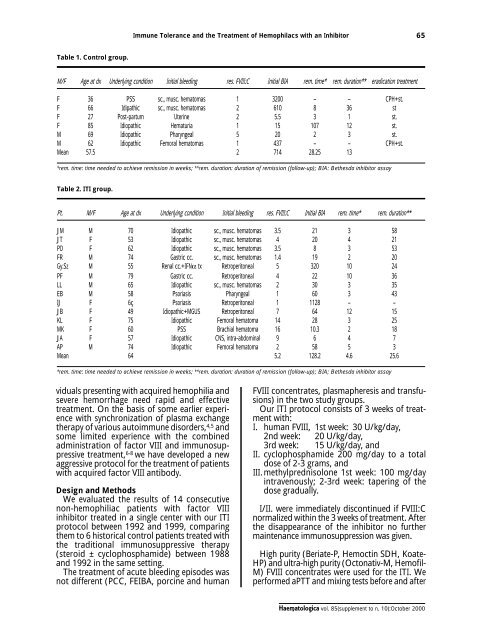
Table 1. Control group.<br />
M/F Age at dx Underlying condition Initial bleeding res. FVIII:C Initial BIA rem. time* rem. duration** eradication treatment<br />
F 36 PSS sc., musc. hema<strong>to</strong>mas 1 3200 – – CPH+st.<br />
F 66 Idipathic sc., musc. hema<strong>to</strong>mas 2 6<strong>10</strong> 8 36 st<br />
F 27 Post-partum Uterine 2 5.5 3 1 st.<br />
F <strong>85</strong> Idiopathic Hematuria 1 15 <strong>10</strong>7 12 st.<br />
M 69 Idiopathic Pharyngeal 5 20 2 3 st.<br />
M 62 Idiopathic Femoral hema<strong>to</strong>mas 1 437 – – CPH+st.<br />
Mean 57.5 2 714 28.25 13<br />
*rem. time: time needed <strong>to</strong> achieve remission in weeks; **rem. duration: duration of remission (follow-up); BIA: Bethesda inhibi<strong>to</strong>r assay<br />
Table 2. ITI group.<br />
Pt. M/F Age at dx Underlying condition Initial bleeding res. FVIII:C Initial BIA rem. time* rem. duration**<br />
JM M 70 Idiopathic sc., musc. hema<strong>to</strong>mas 3.5 21 3 58<br />
JT F 53 Idiopathic sc., musc. hema<strong>to</strong>mas 4 20 4 21<br />
PD F 62 Idiopathic sc., musc. hema<strong>to</strong>mas 3.5 8 3 53<br />
FR M 74 Gastric cc. sc., musc. hema<strong>to</strong>mas 1.4 19 2 20<br />
Gy.Sz M 55 Renal cc.+IFNα tx Retroperi<strong>to</strong>neal 5 320 <strong>10</strong> 24<br />
PF M 79 Gastric cc. Retroperi<strong>to</strong>neal 4 22 <strong>10</strong> 36<br />
LL M 65 Idiopathic sc., musc. hema<strong>to</strong>mas 2 30 3 35<br />
EB M 58 Psoriasis Pharyngeal 1 60 3 43<br />
IJ F 6ç Psoriasis Retroperi<strong>to</strong>neal 1 1128 – –<br />
JB F 49 Idiopathic+MGUS Retroperi<strong>to</strong>neal 7 64 12 15<br />
KL F 75 Idiopathic Femoral hema<strong>to</strong>ma 14 28 3 25<br />
MK F 60 PSS Brachial hema<strong>to</strong>ma 16 <strong>10</strong>.3 2 18<br />
JA F 57 Idiopathic CNS, intra-abdominal 9 6 4 7<br />
AP M 74 Idiopathic Femoral hema<strong>to</strong>ma 2 58 5 3<br />
Mean 64 5.2 128.2 4.6 25.6<br />
*rem. time: time needed <strong>to</strong> achieve remission in weeks; **rem. duration: duration of remission (follow-up); BIA; Bethesda inhibi<strong>to</strong>r assay<br />
viduals presenting with acquired hemophilia and<br />
severe hemorrhage need rapid and effective<br />
treatment. On the basis of some earlier experience<br />
with synchronization of plasma exchange<br />
therapy of various au<strong>to</strong>immune disorders, 4,5 and<br />
some limited experience with the combined<br />
administration of fac<strong>to</strong>r VIII and immu<strong>no</strong>suppressive<br />
treatment, 6-8 we have developed a new<br />
aggressive pro<strong>to</strong>col for the treatment of patients<br />
with acquired fac<strong>to</strong>r VIII antibody.<br />
Design and Methods<br />
We evaluated the results of 14 consecutive<br />
<strong>no</strong>n-hemophiliac patients with fac<strong>to</strong>r VIII<br />
inhibi<strong>to</strong>r treated in a single center with our ITI<br />
pro<strong>to</strong>col between 1992 and 1999, comparing<br />
them <strong>to</strong> 6 his<strong>to</strong>rical control patients treated with<br />
the traditional immu<strong>no</strong>suppressive therapy<br />
(steroid ± cyclophosphamide) between 1988<br />
and 1992 in the same setting.<br />
The treatment of acute bleeding episodes was<br />
<strong>no</strong>t different (PCC, FEIBA, porcine and human<br />
FVIII concentrates, plasmapheresis and transfusions)<br />
in the two study groups.<br />
Our ITI pro<strong>to</strong>col consists of 3 weeks of treatment<br />
with:<br />
I. human FVIII, 1st week: 30 U/kg/day,<br />
2nd week: 20 U/kg/day,<br />
3rd week: 15 U/kg/day, and<br />
II. cyclophosphamide 200 mg/day <strong>to</strong> a <strong>to</strong>tal<br />
dose of 2-3 grams, and<br />
III.methylprednisolone 1st week: <strong>10</strong>0 mg/day<br />
intrave<strong>no</strong>usly; 2-3rd week: tapering of the<br />
dose gradually.<br />
I/II. were immediately discontinued if FVIII:C<br />
<strong>no</strong>rmalized within the 3 weeks of treatment. After<br />
the disappearance of the inhibi<strong>to</strong>r <strong>no</strong> further<br />
maintenance immu<strong>no</strong>suppression was given.<br />
High purity (Beriate-P, Hemoctin SDH, Koate-<br />
HP) and ultra-high purity (Oc<strong>to</strong>nativ-M, Hemofil-<br />
M) FVIII concentrates were used for the ITI. We<br />
performed aPTT and mixing tests before and after<br />
<strong>Haema<strong>to</strong>logica</strong> vol. <strong>85</strong>(<strong>supplement</strong> <strong>to</strong> n. <strong>10</strong>):Oc<strong>to</strong>ber <strong>2000</strong>