TCAs versus placebo - National Center for Biotechnology Information
TCAs versus placebo - National Center for Biotechnology Information
TCAs versus placebo - National Center for Biotechnology Information
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
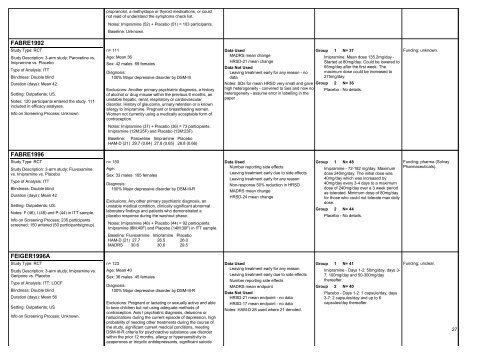
FABRE1992<br />
Study Type: RCT<br />
Study Description: 3-arm study; Paroxetine vs.<br />
Imipramine vs. Placebo<br />
Type of Analysis: ITT<br />
Blindness: Double blind<br />
Duration (days): Mean 42<br />
Setting: Outpatients; US.<br />
Notes: 120 participants entered the study. 111<br />
included in efficacy analyses.<br />
Info on Screening Process: Unknown.<br />
FABRE1996<br />
Study Type: RCT<br />
Study Description: 3-arm study; Fluvoxamine<br />
vs. Imipramine vs. Placebo<br />
Type of Analysis: ITT<br />
Blindness: Double blind<br />
Duration (days): Mean 42<br />
Setting: Outpatients; US.<br />
Notes: F (46), I (48) and P (44) in ITT sample.<br />
Info on Screening Process: 235 participants<br />
screened; 150 entered (50 participants/group).<br />
FEIGER1996A<br />
Study Type: RCT<br />
Study Description: 3-arm study; Imipramine vs.<br />
Geripone vs. Placebo<br />
Type of Analysis: ITT; LOCF<br />
Blindness: Double blind<br />
Duration (days): Mean 56<br />
Setting: Outpatients; US<br />
Info on Screening Process: Unknown.<br />
propranolol, a methyldopa or thyroid medications, or could<br />
not read of understand the symptoms check list.<br />
Notes: Imipramine (52) + Placebo (51) = 103 participants.<br />
Baseline: Unknown.<br />
n= 111<br />
Age: Mean 36<br />
Sex: 42 males 69 females<br />
Diagnosis:<br />
100% Major depressive disorder by DSM-III<br />
Exclusions: Another primary psychiatric diagnosis, a history<br />
of alcohol or drug misuse within the previous 6 months, an<br />
unstable hepatic, renal, respiratory or cardiovascular<br />
disorder. History of glaucoma, urinary retention or a known<br />
allergy to imipramine. Pregnant or breastfeeding women.<br />
Women not currently using a medically acceptable <strong>for</strong>m of<br />
contraception.<br />
Notes: Imipramine (37) + Placebo (36) = 73 participants.<br />
Imipramine (12M:25F) and Placebo (13M:23F).<br />
Baseline: Paroxetine Imipramine Placebo<br />
HAM-D (21) 29.7 (0.64) 27.8 (0.65) 28.8 (0.66)<br />
n= 150<br />
Age:<br />
Sex: 33 males 105 females<br />
Diagnosis:<br />
100% Major depressive disorder by DSM-III-R<br />
Exclusions: Any other primary psychiatric diagnosis, an<br />
unstable medical condition, clinically significant abnormal<br />
laboratory findings and patients who demonstrated a<br />
<strong>placebo</strong> response during the washout phase.<br />
Notes: Imipramine (48) + Placebo (44) = 92 participants.<br />
Imipramine (8M:40F) and Placebo (14M:30F) in ITT sample.<br />
Baseline: Fluvoxamine Imipramine Placebo<br />
HAM-D (21) 27.7 26.5 26.0<br />
MADRS 30.6 30.6 29.5<br />
n= 123<br />
Age: Mean 40<br />
Sex: 36 males 45 females<br />
Diagnosis:<br />
100% Major depressive disorder by DSM-III-R<br />
Exclusions: Pregnant or lactating or sexually active and able<br />
to bear children but not using adequate methods of<br />
contraception. Axis I psychiatric diagnosis, delusions or<br />
hallucinations during the current episode of depression, high<br />
probability of needing other treatments during the course of<br />
the study, significant current medical conditions, meeting<br />
DSM-III-R criteria <strong>for</strong> psychoactive substance use disorder<br />
within the prior 12 months, allergy or hypersensitivity to<br />
azaperones or tricyclic antidepressants, significant suicide<br />
Data Used<br />
MADRS mean change<br />
HRSD-21 mean change<br />
Data Not Used<br />
Leaving treatment early <strong>for</strong> any reason - no<br />
data<br />
Notes: SDs <strong>for</strong> mean HRSD very small and gave<br />
high heterogeneity - convered to Ses and now no<br />
heterogeneity - assume error in labelling in the<br />
paper<br />
Data Used<br />
Number reporting side effects<br />
Leaving treatment early due to side effects<br />
Leaving treatment early <strong>for</strong> any reason<br />
Non-response 50% reduction in HRSD<br />
MADRS mean change<br />
HRSD-24 mean change<br />
Data Used<br />
Leaving treatment early <strong>for</strong> any reason<br />
Leaving treatment early due to side effects<br />
Number reporting side effects<br />
MADRS mean endpoint<br />
Data Not Used<br />
HRSD-21 mean endpoint - no data<br />
HRSD-17 mean endpoint - no data<br />
Notes: HAM-D 28 used where 21 denoted.<br />
Group<br />
Group<br />
Group<br />
Group<br />
Group<br />
Group<br />
1 N= 37<br />
Imipramine. Mean dose 135.2mg/day -<br />
Started at 80mg/day. Could be lowered to<br />
65mg/day after the first week. The<br />
maximum dose could be increased to<br />
275mg/day.<br />
2 N= 36<br />
Placebo - No details.<br />
1 N= 48<br />
Imipramine - 72-182 mg/day. Maximum<br />
dose 240mg/day. The initial dose was<br />
40mg/day which was increased by<br />
40mg/day every 3-4 days to a maximum<br />
dose of 240mg/day over a 3 week period<br />
as tolerated. Minimum dose of 80mg/day<br />
<strong>for</strong> those who could not tolerate max daily<br />
dose.<br />
2 N= 44<br />
Placebo - No details.<br />
1 N= 41<br />
Imipramine - Days 1-2: 50mg/day, days 3-<br />
7: 100mg/day and 50-300mg/day<br />
thereafter.<br />
2 N= 40<br />
Placebo - Days 1-2: 1 capsule/day, days<br />
3-7: 2 capsules/day and up to 6<br />
capsules/day thereafter.<br />
Funding; unknown.<br />
Funding; pharma (Solvay<br />
Pharmaceuticals).<br />
Funding; unclear.<br />
27