TCAs versus placebo - National Center for Biotechnology Information
TCAs versus placebo - National Center for Biotechnology Information
TCAs versus placebo - National Center for Biotechnology Information
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
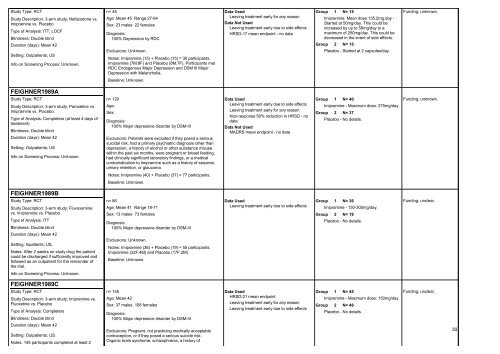
Study Type: RCT<br />
Study Description: 3-arm study; Nefazodone vs.<br />
Imipramine vs. Placebo<br />
Type of Analysis: ITT; LOCF<br />
Blindness: Double blind<br />
Duration (days): Mean 42<br />
Setting: Outpatients; US<br />
Info on Screening Process: Unknown.<br />
FEIGHNER1989A<br />
Study Type: RCT<br />
Study Description: 3-arm study; Paroxetine vs.<br />
Imipramine vs. Placebo.<br />
Type of Analysis: Completers (at least 4 days of<br />
treatment)<br />
Blindness: Double blind<br />
Duration (days): Mean 42<br />
Setting: Outpatients; US<br />
Info on Screening Process: Unknown.<br />
FEIGHNER1989B<br />
Study Type: RCT<br />
Study Description: 3-arm study; Fluvoxamine<br />
vs. Imipramine vs. Placebo<br />
Type of Analysis: ITT<br />
Blindness: Double blind<br />
Duration (days): Mean 42<br />
Setting: Inpatients; US.<br />
Notes: After 2 weeks on study drug the patient<br />
could be discharged if sufficiently improved and<br />
followed as an outpatient <strong>for</strong> the remainder of<br />
the trial.<br />
Info on Screening Process: Unknown.<br />
FEIGHNER1989C<br />
Study Type: RCT<br />
Study Description: 3-arm study; Imipramine vs.<br />
Fluoxetine vs. Placebo<br />
Type of Analysis: Completers<br />
Blindness: Double blind<br />
Duration (days): Mean 42<br />
Setting: Outpatients; US.<br />
Notes: 145 participants completed at least 2<br />
n= 45<br />
Age: Mean 45 Range 27-64<br />
Sex: 23 males 22 females<br />
Diagnosis:<br />
100% Depression by RDC<br />
Exclusions: Unknown.<br />
Notes: Imipramine (15) + Placebo (15) = 30 participants.<br />
Imipramine (7M:8F) and Placebo (8M:7F). Participants met<br />
RDC Endogenous Major Depression and DSM III Major<br />
Depression with Melancholia.<br />
Baseline: Unknown.<br />
n= 120<br />
Age:<br />
Sex:<br />
Diagnosis:<br />
100% Major depressive disorder by DSM-III<br />
Exclusions: Patients were excluded if they posed a serious<br />
suicidal risk, had a primary psychiatric diagnosis other than<br />
depression, a history of alcohol or other substance misuse<br />
within the past six months, were pregnant or breast feeding,<br />
had clinically significant laboratory findings, or a medical<br />
contraindication to imipramine such as a history of seizures,<br />
urinary retention, or glaucoma.<br />
Notes: Imipramine (40) + Placebo (37) = 77 participants.<br />
Baseline: Unknown.<br />
n= 86<br />
Age: Mean 41 Range 18-71<br />
Sex: 13 males 73 females<br />
Diagnosis:<br />
100% Major depressive disorder by DSM-III<br />
Exclusions: Unknown.<br />
Notes: Imipramine (36) + Placebo (19) = 55 participants.<br />
Imipramine (32F:4M) and Placebo (17F:2M).<br />
Baseline: Unknown.<br />
n= 145<br />
Age: Mean 42<br />
Sex: 37 males 108 females<br />
Diagnosis:<br />
100% Major depressive disorder by DSM-III<br />
Exclusions: Pregnant, not practicing medically acceptable<br />
contraception, or if they posed a serious suicide risk.<br />
Organic brain syndrome, schizophrenia, a history of<br />
Data Used<br />
Leaving treatment early <strong>for</strong> any reason<br />
Data Not Used<br />
Leaving treatment early due to side effects<br />
HRSD-17 mean endpoint - no data<br />
Data Used<br />
Leaving treatment early due to side effects<br />
Leaving treatment early <strong>for</strong> any reason<br />
Non-response 50% reduction in HRSD - no<br />
data<br />
Data Not Used<br />
MADRS mean endpoint - no data<br />
Data Used<br />
Leaving treatment early due to side effects<br />
Data Used<br />
HRSD-21 mean endpoint<br />
Leaving treatment early <strong>for</strong> any reason<br />
Leaving treatment early due to side effects<br />
Group<br />
Group<br />
Group<br />
Group<br />
Group<br />
Group<br />
Group<br />
Group<br />
1 N= 15<br />
Imipramine. Mean dose 135.2mg.day -<br />
Started at 50mg/day. This could be<br />
increased by up to 50mg/day to a<br />
maximum of 250mg/day. This could be<br />
decreased in the event of side effects.<br />
2 N= 15<br />
Placebo - Started at 2 capsules/day.<br />
1 N= 40<br />
Imipramine - Maximum dose: 275mg/day.<br />
2 N= 37<br />
Placebo - No details.<br />
1 N= 36<br />
Imipramine - 150-300mg/day.<br />
2 N= 19<br />
Placebo - No details.<br />
1 N= 45<br />
Imipramine - Maximum dose: 150mg/day.<br />
2 N= 48<br />
Placebo - No details.<br />
Funding; unknown.<br />
Funding; unknown.<br />
Funding; unclear.<br />
Funding; unclear.<br />
30