TCAs versus placebo - National Center for Biotechnology Information
TCAs versus placebo - National Center for Biotechnology Information
TCAs versus placebo - National Center for Biotechnology Information
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
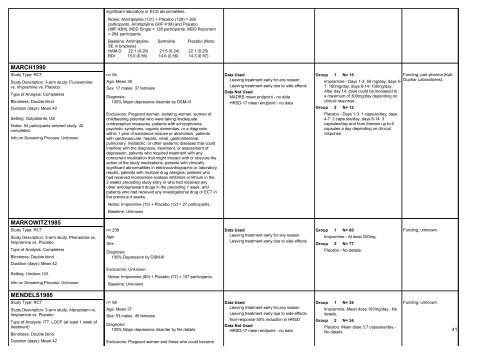
MARCH1990<br />
Study Type: RCT<br />
Study Description: 3-arm study; Fluvoxamine<br />
vs. Imipramine vs. Placebo<br />
Type of Analysis: Completers<br />
Blindness: Double blind<br />
Duration (days): Mean 42<br />
Setting: Outpatients; US<br />
Notes: 54 participants entered study. 40<br />
completed.<br />
Info on Screening Process: Unknown.<br />
MARKOWITZ1985<br />
Study Type: RCT<br />
Study Description: 3-arm study; Phenelzine vs.<br />
Imipramine vs. Placebo<br />
Type of Analysis: Completers<br />
Blindness: Double blind<br />
Duration (days): Mean 42<br />
Setting: Unclear; US.<br />
Info on Screening Process: Unknown.<br />
MENDELS1986<br />
Study Type: RCT<br />
Study Description: 3-arm study; Alprazolam vs.<br />
Imipramine vs. Placebo<br />
Type of Analysis: ITT: LOCF (at least 1 week of<br />
treatment)<br />
Blindness: Double blind<br />
Duration (days): Mean 42<br />
significant laboratory or ECG abnormalities.<br />
Notes: Amitriptyline (131) + Placebo (129) = 260<br />
participants. Amitriptyline (90F:41M) and Placebo<br />
(86F:43M). MDD Single = 128 participants. MDD Recurrent<br />
= 264 participants.<br />
Baseline: Amitriptyline Sertraline Placebo (Note:<br />
SE in brackets)<br />
HAM-D 22.1 (0.26) 21.5 (0.24) 22.1 (0.25)<br />
BDI 15.0 (0.56) 14.6 (0.56) 14.3 (0.57)<br />
n= 54<br />
Age: Mean 39<br />
Sex: 17 males 37 females<br />
Diagnosis:<br />
100% Major depressive disorder by DSM-III<br />
Exclusions: Pregnant women, lactating women, women of<br />
childbearing potential who were taking inadequate<br />
contraceptive measures, patients with schizophrenia,<br />
psychotic symptoms, organic dementias, or a diagnosis<br />
within 1 year of substance misuse or alcoholism, patients<br />
with cardiovascular, hepatic, renal, gastrointestinal,<br />
pulmonary, metabolic, or other systemic diseases that could<br />
interfere with the diagnosis, treatment, or assessment of<br />
depression, patients who required treatment with any<br />
concurrent medication that might interact with or obscure the<br />
action of the study medications, patients with clinically<br />
significant abnormalities in electrocardiographic or laboratory<br />
results, patients with multiple drug allergies, patients who<br />
had received monoamine oxidase inhibitors or lithium in the<br />
2 weeks preceding study entry or who had received any<br />
other antidepressant drugs in the preceding 1 week, and<br />
patients who had received any investigational drug or ECT in<br />
the previous 4 weeks.<br />
Notes: Imipramine (15) + Placebo (12) = 27 participants.<br />
Baseline: Unknown.<br />
n= 238<br />
Age:<br />
Sex:<br />
Diagnosis:<br />
100% Depression by DSM-III<br />
Exclusions: Unknown.<br />
Notes: Imipramine (80) + Placebo (77) = 157 participants.<br />
Baseline: Unknown.<br />
n= 98<br />
Age: Mean 37<br />
Data Used<br />
Leaving treatment early <strong>for</strong> any reason<br />
Leaving treatment early due to side effects<br />
Data Not Used<br />
MADRS mean endpoint - no data<br />
HRSD-17 mean endpoint - no data<br />
Data Used<br />
Leaving treatment early <strong>for</strong> any reason<br />
Leaving treatment early due to side effects<br />
Data Used<br />
Leaving treatment early <strong>for</strong> any reason<br />
Leaving treatment early due to side effects<br />
Non-response 50% reduction in HRSD<br />
Data Not Used<br />
HRSD-17 mean endpoint - no data<br />
Group<br />
Group<br />
Group<br />
Group<br />
Group<br />
1 N= 15<br />
Imipramine - Days 1-3: 50 mg/day, days 4-<br />
7: 100mg/day, days 8-14: 150mg/day.<br />
After day 14, dose could be increased to<br />
a maximum of 300mg/day depending on<br />
clinical response.<br />
2 N= 12<br />
Placebo - Days 1-3: 1 capsule/day, days<br />
4-7: 2 capsules/day, days 8-14: 3<br />
capsules/day and from thereon up to 6<br />
capsules a day depending on clinical<br />
response.<br />
1 N= 80<br />
Imipramine - At least 200mg.<br />
2 N= 77<br />
Placebo - No details.<br />
1 N= 34<br />
Imipramine. Mean dose 167mg/day - No<br />
details.<br />
Funding; part-pharma (Kali-<br />
Duphar Laboratories).<br />
Funding; unknown.<br />
Funding; unknown.<br />
Sex: 53 males 45 females<br />
Diagnosis:<br />
100% Major depressive disorder by No details<br />
Group 2 N= 34<br />
Placebo. Mean dose 3.7 capsules/day -<br />
No details.<br />
41<br />
Exclusions: Pregnant women and those who could become