TCAs versus placebo - National Center for Biotechnology Information
TCAs versus placebo - National Center for Biotechnology Information
TCAs versus placebo - National Center for Biotechnology Information
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
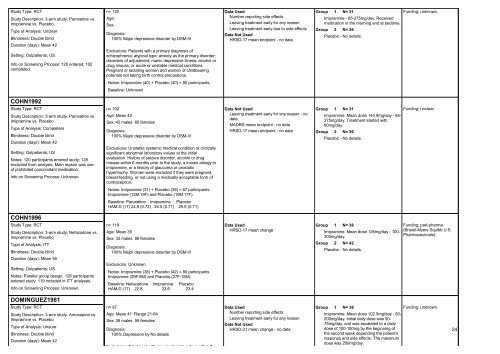
Study Type: RCT<br />
Study Description: 3-arm study; Paroxetine vs.<br />
Imipramine vs. Placebo.<br />
Type of Analysis: Unclear<br />
Blindness: Double blind<br />
Duration (days): Mean 42<br />
Setting: Outpatients; US.<br />
Info on Screening Process: 120 entered; 102<br />
completed.<br />
COHN1992<br />
Study Type: RCT<br />
Study Description: 3-arm study; Paroxetine vs.<br />
Imipramine vs. Placebo<br />
Type of Analysis: Completers<br />
Blindness: Double blind<br />
Duration (days): Mean 42<br />
Setting: Outpatients; US<br />
Notes: 120 participants entered study; 128<br />
excluded from analysis. Main reason was use<br />
of prohibited concomitant medication.<br />
Info on Screening Process: Unknown.<br />
COHN1996<br />
Study Type: RCT<br />
Study Description: 3-arm study; Nefazodone vs.<br />
Imipramine vs. Placebo<br />
Type of Analysis: ITT<br />
Blindness: Double blind<br />
Duration (days): Mean 56<br />
Setting: Outpatients; US.<br />
Notes: Parallel group design. 128 participants<br />
entered study; 119 included in ITT analyses.<br />
Info on Screening Process: Unknown.<br />
DOMINGUEZ1981<br />
Study Type: RCT<br />
Study Description: 3-arm study; Amoxapine vs.<br />
Imipramine vs. Placebo<br />
Type of Analysis: Unsure<br />
Blindness: Double blind<br />
Duration (days): Mean 42<br />
n= 120<br />
Age:<br />
Sex:<br />
Diagnosis:<br />
100% Major depressive disorder by DSM-III<br />
Exclusions: Patients with a primary diagnosis of<br />
schizophrenia; atypical type; anxiety as the primary disorder;<br />
disorders of adjustment; manic depressive illness; alcohol or<br />
drug misuse; or acute or unstable medical conditions.<br />
Pregnant or lactating women and women of childbearing<br />
potential not taking birth control precautions.<br />
Notes: Imipramine (40) + Placebo (40) = 80 participants.<br />
Baseline: Unknown.<br />
n= 102<br />
Age: Mean 42<br />
Sex: 42 males 60 females<br />
Diagnosis:<br />
100% Major depressive disorder by DSM-III<br />
Exclusions: Unstable systemic medical condition or clinically<br />
significant abnormal laboratory values at the initial<br />
evaluation. History of seizure disorder, alcohol or drug<br />
misuse within 6 months prior to the study, a known allergy to<br />
imipramine, or a history of glaucoma or prostatic<br />
hypertrophy. Women were excluded if they were pregnant,<br />
breast-feeding, or not using a medically acceptable <strong>for</strong>m of<br />
contraception.<br />
Notes: Imipramine (31) + Placebo (36) = 67 participants.<br />
Imipramine (12M:19F) and Placebo (19M:17F).<br />
Baseline: Paroxetine Imipramine Placebo<br />
HAM-D (17) 24.9 (0.72) 24.5 (0.71) 25.6 (0.71)<br />
n= 119<br />
Age: Mean 39<br />
Sex: 33 males 86 females<br />
Diagnosis:<br />
100% Major depressive disorder by DSM-III<br />
Exclusions: Unknown.<br />
Notes: Imipramine (38) + Placebo (42) = 80 participants.<br />
Imipramine (29F:9M) and Placebo (27F:15M).<br />
Baseline: Nefazadone Imipramine Placebo<br />
HAM-D (17) 22.8 23.6 23.4<br />
n= 97<br />
Age: Mean 41 Range 21-64<br />
Sex: 38 males 59 females<br />
Diagnosis:<br />
100% Depression by No details<br />
Exclusions: Treatment with any psychoactive drug within 7<br />
Data Used<br />
Number reporting side effects<br />
Leaving treatment early <strong>for</strong> any reason<br />
Leaving treatment early due to side effects<br />
Data Not Used<br />
HRSD-17 mean endpoint - no data<br />
Data Not Used<br />
Leaving treatment early <strong>for</strong> any reason - no<br />
data<br />
MADRS mean endpoint - no data<br />
HRSD-17 mean endpoint - no data<br />
Data Used<br />
HRSD-17 mean change<br />
Data Used<br />
Number reporting side effects<br />
Leaving treatment early <strong>for</strong> any reason<br />
Data Not Used<br />
HRSD-21 mean change - no data<br />
Group<br />
Group<br />
Group<br />
Group<br />
Group<br />
Group<br />
Group<br />
1 N= 31<br />
Imipramine - 65-275mg/day. Received<br />
medication in the morning and at bedtime.<br />
2 N= 36<br />
Placebo - No details.<br />
1 N= 31<br />
Imipramine. Mean dose 144.9mg/day - 65-<br />
275mg/day. Treatment started with<br />
80mg/day.<br />
2 N= 36<br />
Placebo - No details.<br />
1 N= 38<br />
Imipramine. Mean dose 126mg/day - 100-<br />
300mg/day.<br />
2 N= 42<br />
Placebo - No details.<br />
1 N= 38<br />
Imipramine. Mean dose 102.5mg/day - 50-<br />
200mg/day. Initial daily dose was 50-<br />
75mg/day, and was escalated to a daily<br />
dose of 100-150mg by the beginning of<br />
the second week depending the patient's<br />
response and side effects. The maximum<br />
dose was 200mg/day.<br />
Funding; unknown.<br />
Funding; unclear.<br />
Funding; part-pharma<br />
(Bristol-Myers Squibb U.S.<br />
Pharmaceuticals).<br />
Funding; unknown.<br />
24