Statistical thermodynamics 1: the concepts - W.H. Freeman
Statistical thermodynamics 1: the concepts - W.H. Freeman
Statistical thermodynamics 1: the concepts - W.H. Freeman
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
PC8eC16 1/26/06 14:34 Page 578<br />
578 16 STATISTICAL THERMODYNAMICS 1: THE CONCEPTS<br />
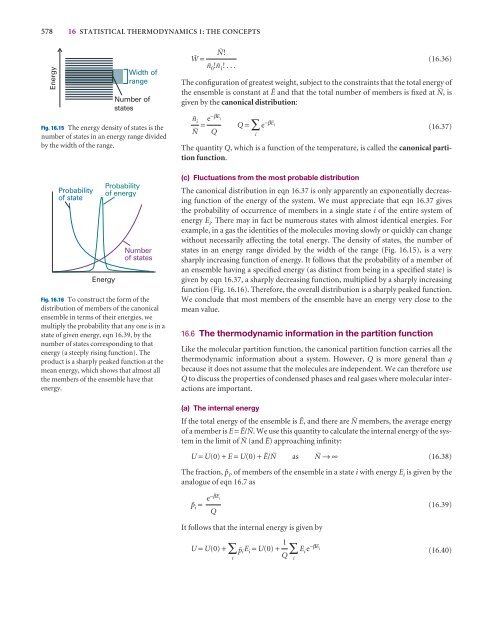
Energy<br />
Width of<br />
range<br />
Number of<br />
states<br />
Fig. 16.15 The energy density of states is <strong>the</strong><br />
number of states in an energy range divided<br />
by <strong>the</strong> width of <strong>the</strong> range.<br />
Probability<br />
of state<br />
Probability<br />
of energy<br />
Energy<br />
Number<br />
of states<br />
Fig. 16.16 To construct <strong>the</strong> form of <strong>the</strong><br />
distribution of members of <strong>the</strong> canonical<br />
ensemble in terms of <strong>the</strong>ir energies, we<br />
multiply <strong>the</strong> probability that any one is in a<br />
state of given energy, eqn 16.39, by <strong>the</strong><br />
number of states corresponding to that<br />
energy (a steeply rising function). The<br />
product is a sharply peaked function at <strong>the</strong><br />
mean energy, which shows that almost all<br />
<strong>the</strong> members of <strong>the</strong> ensemble have that<br />
energy.<br />
Ñ!<br />
M = (16.36)<br />
ñ 0 !ñ 1 ! . . .<br />
The configuration of greatest weight, subject to <strong>the</strong> constraints that <strong>the</strong> total energy of<br />
<strong>the</strong> ensemble is constant at L and that <strong>the</strong> total number of members is fixed at Ñ, is<br />
given by <strong>the</strong> canonical distribution:<br />
ñ e −βE i i<br />
= Q = ∑ e −βE i<br />
(16.37)<br />
Ñ Q<br />
i<br />
The quantity Q, which is a function of <strong>the</strong> temperature, is called <strong>the</strong> canonical partition<br />
function.<br />
(c) Fluctuations from <strong>the</strong> most probable distribution<br />
The canonical distribution in eqn 16.37 is only apparently an exponentially decreasing<br />
function of <strong>the</strong> energy of <strong>the</strong> system. We must appreciate that eqn 16.37 gives<br />
<strong>the</strong> probability of occurrence of members in a single state i of <strong>the</strong> entire system of<br />
energy E i . There may in fact be numerous states with almost identical energies. For<br />
example, in a gas <strong>the</strong> identities of <strong>the</strong> molecules moving slowly or quickly can change<br />
without necessarily affecting <strong>the</strong> total energy. The density of states, <strong>the</strong> number of<br />
states in an energy range divided by <strong>the</strong> width of <strong>the</strong> range (Fig. 16.15), is a very<br />
sharply increasing function of energy. It follows that <strong>the</strong> probability of a member of<br />
an ensemble having a specified energy (as distinct from being in a specified state) is<br />
given by eqn 16.37, a sharply decreasing function, multiplied by a sharply increasing<br />
function (Fig. 16.16). Therefore, <strong>the</strong> overall distribution is a sharply peaked function.<br />
We conclude that most members of <strong>the</strong> ensemble have an energy very close to <strong>the</strong><br />
mean value.<br />
16.6 The <strong>the</strong>rmodynamic information in <strong>the</strong> partition function<br />
Like <strong>the</strong> molecular partition function, <strong>the</strong> canonical partition function carries all <strong>the</strong><br />
<strong>the</strong>rmodynamic information about a system. However, Q is more general than q<br />
because it does not assume that <strong>the</strong> molecules are independent. We can <strong>the</strong>refore use<br />
Q to discuss <strong>the</strong> properties of condensed phases and real gases where molecular interactions<br />
are important.<br />
(a) The internal energy<br />
If <strong>the</strong> total energy of <strong>the</strong> ensemble is L, and <strong>the</strong>re are Ñ members, <strong>the</strong> average energy<br />
of a member is E = L/Ñ. We use this quantity to calculate <strong>the</strong> internal energy of <strong>the</strong> system<br />
in <strong>the</strong> limit of Ñ (and L) approaching infinity:<br />
U = U(0) + E = U(0) + L/Ñ as Ñ →∞ (16.38)<br />
The fraction, " i , of members of <strong>the</strong> ensemble in a state i with energy E i is given by <strong>the</strong><br />
analogue of eqn 16.7 as<br />
e −βE i<br />
" i = (16.39)<br />
Q<br />
It follows that <strong>the</strong> internal energy is given by<br />
U = U(0) + ∑<br />
i<br />
"i E i = U(0) + 1<br />
∑ E i e −βE i<br />
(16.40)<br />
Q<br />
i