Statistical thermodynamics 1: the concepts - W.H. Freeman
Statistical thermodynamics 1: the concepts - W.H. Freeman
Statistical thermodynamics 1: the concepts - W.H. Freeman
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
PC8eC16 1/26/06 14:34 Page 585<br />
DISCUSSION QUESTIONS 585<br />
6<br />
1<br />
Partition function, q<br />
4<br />
2<br />
Entropy, SNk /<br />
0.5<br />
10<br />
5<br />
0<br />
/kT<br />
5 10<br />
0 0.5 1<br />
Internal energy, UN / <br />
<br />
Internal energy, UN /<br />
Entropy., SNk /<br />
1<br />
0.5<br />
10<br />
1<br />
0.5<br />
10<br />
5<br />
5<br />
0<br />
/kT<br />
0<br />
/kT<br />
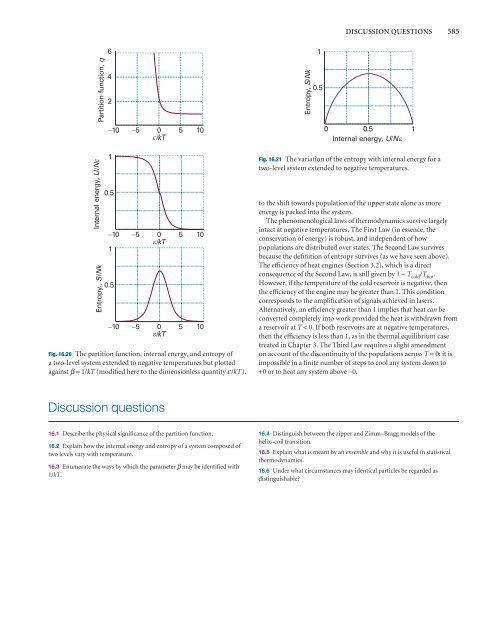
Fig. 16.20 The partition function, internal energy, and entropy of<br />
a two-level system extended to negative temperatures but plotted<br />
against β = 1/kT (modified here to <strong>the</strong> dimensionless quantity ε/kT).<br />
5<br />
5<br />
10<br />
10<br />
Fig. 16.21 The variation of <strong>the</strong> entropy with internal energy for a<br />
two-level system extended to negative temperatures.<br />
to <strong>the</strong> shift towards population of <strong>the</strong> upper state alone as more<br />
energy is packed into <strong>the</strong> system.<br />
The phenomenological laws of <strong><strong>the</strong>rmodynamics</strong> survive largely<br />
intact at negative temperatures. The First Law (in essence, <strong>the</strong><br />
conservation of energy) is robust, and independent of how<br />
populations are distributed over states. The Second Law survives<br />
because <strong>the</strong> definition of entropy survives (as we have seen above).<br />
The efficiency of heat engines (Section 3.2), which is a direct<br />
consequence of <strong>the</strong> Second Law, is still given by 1 − T cold /T hot .<br />
However, if <strong>the</strong> temperature of <strong>the</strong> cold reservoir is negative, <strong>the</strong>n<br />
<strong>the</strong> efficiency of <strong>the</strong> engine may be greater than 1. This condition<br />
corresponds to <strong>the</strong> amplification of signals achieved in lasers.<br />
Alternatively, an efficiency greater than 1 implies that heat can be<br />
converted completely into work provided <strong>the</strong> heat is withdrawn from<br />
a reservoir at T < 0. If both reservoirs are at negative temperatures,<br />
<strong>the</strong>n <strong>the</strong> efficiency is less than 1, as in <strong>the</strong> <strong>the</strong>rmal equilibrium case<br />
treated in Chapter 3. The Third Law requires a slight amendment<br />
on account of <strong>the</strong> discontinuity of <strong>the</strong> populations across T = 0: it is<br />
impossible in a finite number of steps to cool any system down to<br />
+0 or to heat any system above −0.<br />
Discussion questions<br />
16.1 Describe <strong>the</strong> physical significance of <strong>the</strong> partition function.<br />
16.2 Explain how <strong>the</strong> internal energy and entropy of a system composed of<br />
two levels vary with temperature.<br />
16.3 Enumerate <strong>the</strong> ways by which <strong>the</strong> parameter β may be identified with<br />
1/kT.<br />
16.4 Distinguish between <strong>the</strong> zipper and Zimm–Bragg models of <strong>the</strong><br />
helix–coil transition.<br />
16.5 Explain what is meant by an ensemble and why it is useful in statistical<br />
<strong><strong>the</strong>rmodynamics</strong>.<br />
16.6 Under what circumstances may identical particles be regarded as<br />
distinguishable