Polyatomic molecules - Cobalt
Polyatomic molecules - Cobalt
Polyatomic molecules - Cobalt
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
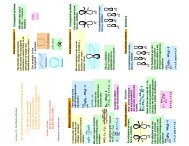
2. (a) Consider the allyl molecule CH 2CHCH2.<br />
Construct<br />
the secular determinant and find the orbital energies for the<br />
π - electron system. Find the molecular orbitals and scetch them.<br />
You can make use of the results from the previous question on H3<br />
(b) Do the same for cyclopropene<br />
H<br />
C<br />
H 2 C<br />
CH 2 HC<br />
H<br />
C<br />
CH<br />
Answer :<br />
For (a) we get the secular determinant<br />
α − E<br />
β α−<br />
E β<br />
β α−<br />
E<br />
= 0<br />
sin ce the two terminal hydrogens C 1 and C3<br />
do not interact<br />
α−<br />
E β<br />
β α−<br />
E β<br />
β α−<br />
E<br />
=<br />
α−<br />
E β<br />
( α − E) ( ) − β β β<br />
β ( α−<br />
E) 0 ( α − E)<br />
3 2 2<br />
= ( α−E) −β ( α−E) −β ( α−E)<br />
2 2<br />
= ( α−E) α−E)<br />
−2β<br />
0<br />
[ ] =<br />
Thus<br />
ε1 = α+ 2β;<br />
ε2 = α;<br />
ε3<br />
= α−<br />
2β