Drugs in the Pipeline Analysis
Drugs in the Pipeline Analysis
Drugs in the Pipeline Analysis
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
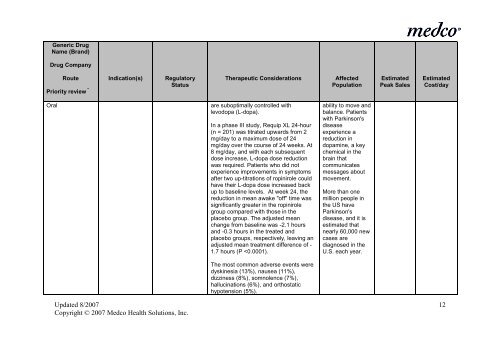
Generic Drug<br />
Name (Brand)<br />
Drug Company<br />
Route Indication(s) Regulatory<br />
Priority review * Status<br />
Therapeutic Considerations<br />
Affected<br />
Population<br />
Estimated<br />
Peak Sales<br />
Estimated<br />
Cost/day<br />
Oral<br />
are suboptimally controlled with<br />
levodopa (L-dopa).<br />
In a phase III study, Requip XL 24-hour<br />
(n = 201) was titrated upwards from 2<br />
mg/day to a maximum dose of 24<br />
mg/day over <strong>the</strong> course of 24 weeks. At<br />
8 mg/day, and with each subsequent<br />
dose <strong>in</strong>crease, L-dopa dose reduction<br />
was required. Patients who did not<br />
experience improvements <strong>in</strong> symptoms<br />
after two up-titrations of rop<strong>in</strong>irole could<br />
have <strong>the</strong>ir L-dopa dose <strong>in</strong>creased back<br />
up to basel<strong>in</strong>e levels. At week 24, <strong>the</strong><br />
reduction <strong>in</strong> mean awake "off" time was<br />
significantly greater <strong>in</strong> <strong>the</strong> rop<strong>in</strong>irole<br />
group compared with those <strong>in</strong> <strong>the</strong><br />
placebo group. The adjusted mean<br />
change from basel<strong>in</strong>e was -2.1 hours<br />
and -0.3 hours <strong>in</strong> <strong>the</strong> treated and<br />
placebo groups, respectively, leav<strong>in</strong>g an<br />
adjusted mean treatment difference of -<br />
1.7 hours (P