Drugs in the Pipeline Analysis
Drugs in the Pipeline Analysis
Drugs in the Pipeline Analysis
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
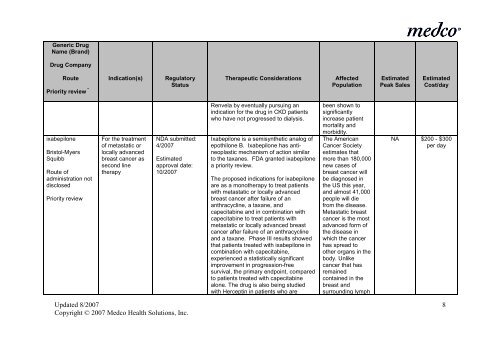
Generic Drug<br />
Name (Brand)<br />
Drug Company<br />
Route Indication(s) Regulatory<br />
Priority review * Status<br />
Therapeutic Considerations<br />
Affected<br />
Population<br />
Estimated<br />
Peak Sales<br />
Estimated<br />
Cost/day<br />
ixabepilone<br />
Bristol-Myers<br />
Squibb<br />
Route of<br />
adm<strong>in</strong>istration not<br />
disclosed<br />
Priority review<br />
For <strong>the</strong> treatment<br />
of metastatic or<br />
locally advanced<br />
breast cancer as<br />
second l<strong>in</strong>e<br />
<strong>the</strong>rapy<br />
NDA submitted:<br />
4/2007<br />
Estimated<br />
approval date:<br />
10/2007<br />
Renvela by eventually pursu<strong>in</strong>g an<br />
<strong>in</strong>dication for <strong>the</strong> drug <strong>in</strong> CKD patients<br />
who have not progressed to dialysis.<br />
Ixabepilone is a semisyn<strong>the</strong>tic analog of<br />
epothilone B. Ixabepilone has ant<strong>in</strong>eoplastic<br />
mechanism of action similar<br />
to <strong>the</strong> taxanes. FDA granted ixabepilone<br />
a priority review.<br />
The proposed <strong>in</strong>dications for ixabepilone<br />
are as a mono<strong>the</strong>rapy to treat patients<br />
with metastatic or locally advanced<br />
breast cancer after failure of an<br />
anthracycl<strong>in</strong>e, a taxane, and<br />
capecitab<strong>in</strong>e and <strong>in</strong> comb<strong>in</strong>ation with<br />
capecitab<strong>in</strong>e to treat patients with<br />
metastatic or locally advanced breast<br />
cancer after failure of an anthracycl<strong>in</strong>e<br />
and a taxane. Phase III results showed<br />
that patients treated with ixabepilone <strong>in</strong><br />
comb<strong>in</strong>ation with capecitab<strong>in</strong>e,<br />
experienced a statistically significant<br />
improvement <strong>in</strong> progression-free<br />
survival, <strong>the</strong> primary endpo<strong>in</strong>t, compared<br />
to patients treated with capecitab<strong>in</strong>e<br />
alone. The drug is also be<strong>in</strong>g studied<br />
with Hercept<strong>in</strong> <strong>in</strong> patients who are<br />
been shown to<br />
significantly<br />
<strong>in</strong>crease patient<br />
mortality and<br />
morbidity.<br />
The American<br />
Cancer Society<br />
estimates that<br />
more than 180,000<br />
new cases of<br />
breast cancer will<br />
be diagnosed <strong>in</strong><br />
<strong>the</strong> US this year,<br />
and almost 41,000<br />
people will die<br />
from <strong>the</strong> disease.<br />
Metastatic breast<br />
cancer is <strong>the</strong> most<br />
advanced form of<br />
<strong>the</strong> disease <strong>in</strong><br />
which <strong>the</strong> cancer<br />
has spread to<br />
o<strong>the</strong>r organs <strong>in</strong> <strong>the</strong><br />
body. Unlike<br />
cancer that has<br />
rema<strong>in</strong>ed<br />
conta<strong>in</strong>ed <strong>in</strong> <strong>the</strong><br />
breast and<br />
surround<strong>in</strong>g lymph<br />
NA $200 - $300<br />
per day<br />
Updated 8/2007<br />
Copyright © 2007 Medco Health Solutions, Inc.<br />
8