Drugs in the Pipeline Analysis
Drugs in the Pipeline Analysis
Drugs in the Pipeline Analysis
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
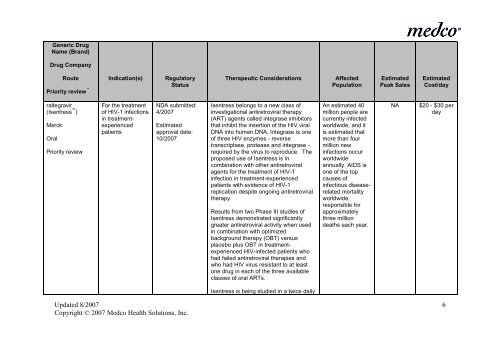
Generic Drug<br />
Name (Brand)<br />
Drug Company<br />
Route Indication(s) Regulatory<br />
Priority review * Status<br />
Therapeutic Considerations<br />
Affected<br />
Population<br />
Estimated<br />
Peak Sales<br />
Estimated<br />
Cost/day<br />
raltegravir<br />
(Isentress )<br />
Merck<br />
Oral<br />
Priority review<br />
For <strong>the</strong> treatment<br />
of HIV-1 <strong>in</strong>fections<br />
<strong>in</strong> treatmentexperienced<br />
patients<br />
NDA submitted:<br />
4/2007<br />
Estimated<br />
approval date:<br />
10/2007<br />
Isentress belongs to a new class of<br />
<strong>in</strong>vestigational antiretroviral <strong>the</strong>rapy<br />
(ART) agents called <strong>in</strong>tegrase <strong>in</strong>hibitors<br />
that <strong>in</strong>hibit <strong>the</strong> <strong>in</strong>sertion of <strong>the</strong> HIV viral<br />
DNA <strong>in</strong>to human DNA. Integrase is one<br />
of three HIV enzymes - reverse<br />
transcriptase, protease and <strong>in</strong>tegrase -<br />
required by <strong>the</strong> virus to reproduce. The<br />
proposed use of Isentress is <strong>in</strong><br />
comb<strong>in</strong>ation with o<strong>the</strong>r antiretroviral<br />
agents for <strong>the</strong> treatment of HIV-1<br />
<strong>in</strong>fection <strong>in</strong> treatment-experienced<br />
patients with evidence of HIV-1<br />
replication despite ongo<strong>in</strong>g antiretroviral<br />
<strong>the</strong>rapy.<br />
Results from two Phase III studies of<br />
Isentress demonstrated significantly<br />
greater antiretroviral activity when used<br />
<strong>in</strong> comb<strong>in</strong>ation with optimized<br />
background <strong>the</strong>rapy (OBT) versus<br />
placebo plus OBT <strong>in</strong> treatmentexperienced<br />
HIV-<strong>in</strong>fected patients who<br />
had failed antiretroviral <strong>the</strong>rapies and<br />
who had HIV virus resistant to at least<br />
one drug <strong>in</strong> each of <strong>the</strong> three available<br />
classes of oral ARTs.<br />
An estimated 40<br />
million people are<br />
currently <strong>in</strong>fected<br />
worldwide, and it<br />
is estimated that<br />
more than four<br />
million new<br />
<strong>in</strong>fections occur<br />
worldwide<br />
annually. AIDS is<br />
one of <strong>the</strong> top<br />
causes of<br />
<strong>in</strong>fectious diseaserelated<br />
mortality<br />
worldwide,<br />
responsible for<br />
approximately<br />
three million<br />
deaths each year.<br />
NA<br />
$20 - $30 per<br />
day<br />
Isentress is be<strong>in</strong>g studied <strong>in</strong> a twice daily<br />
Updated 8/2007<br />
Copyright © 2007 Medco Health Solutions, Inc.<br />
6