Genetic susceptibility to adverse drug effects - Epidemiology ...
Genetic susceptibility to adverse drug effects - Epidemiology ...
Genetic susceptibility to adverse drug effects - Epidemiology ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chapter 5<br />
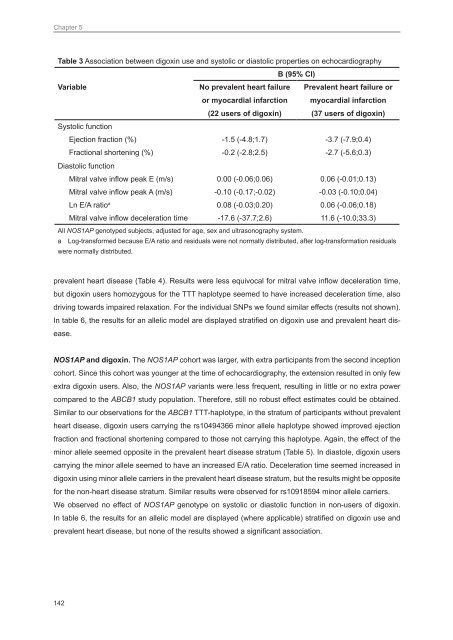
Table 3 Association between digoxin use and sys<strong>to</strong>lic or dias<strong>to</strong>lic properties on echocardiography<br />
B (95% CI)<br />
Variable<br />
No prevalent heart failure<br />
or myocardial infarction<br />
(22 users of digoxin)<br />
Prevalent heart failure or<br />
myocardial infarction<br />
(37 users of digoxin)<br />
Sys<strong>to</strong>lic function<br />
Ejection fraction (%) -1.5 (-4.8;1.7) -3.7 (-7.9;0.4)<br />
Fractional shortening (%) -0.2 (-2.8;2.5) -2.7 (-5.6;0.3)<br />
Dias<strong>to</strong>lic function<br />
Mitral valve inflow peak E (m/s) 0.00 (-0.06;0.06) 0.06 (-0.01;0.13)<br />
Mitral valve inflow peak A (m/s) -0.10 (-0.17;-0.02) -0.03 (-0.10;0.04)<br />
Ln E/A ratio a 0.08 (-0.03;0.20) 0.06 (-0.06;0.18)<br />
Mitral valve inflow deceleration time -17.6 (-37.7;2.6) 11.6 (-10.0;33.3)<br />
All NOS1AP genotyped subjects, adjusted for age, sex and ultrasonography system.<br />
a Log-transformed because E/A ratio and residuals were not normally distributed, after log-transformation residuals<br />
were normally distributed.<br />
prevalent heart disease (Table 4). Results were less equivocal for mitral valve inflow deceleration time,<br />
but digoxin users homozygous for the TTT haplotype seemed <strong>to</strong> have increased deceleration time, also<br />
driving <strong>to</strong>wards impaired relaxation. For the individual SNPs we found similar <strong>effects</strong> (results not shown).<br />
In table 6, the results for an allelic model are displayed stratified on digoxin use and prevalent heart disease.<br />
NOS1AP and digoxin. The NOS1AP cohort was larger, with extra participants from the second inception<br />
cohort. Since this cohort was younger at the time of echocardiography, the extension resulted in only few<br />
extra digoxin users. Also, the NOS1AP variants were less frequent, resulting in little or no extra power<br />
compared <strong>to</strong> the ABCB1 study population. Therefore, still no robust effect estimates could be obtained.<br />
Similar <strong>to</strong> our observations for the ABCB1 TTT-haplotype, in the stratum of participants without prevalent<br />
heart disease, digoxin users carrying the rs10494366 minor allele haplotype showed improved ejection<br />
fraction and fractional shortening compared <strong>to</strong> those not carrying this haplotype. Again, the effect of the<br />
minor allele seemed opposite in the prevalent heart disease stratum (Table 5). In dias<strong>to</strong>le, digoxin users<br />
carrying the minor allele seemed <strong>to</strong> have an increased E/A ratio. Deceleration time seemed increased in<br />
digoxin using minor allele carriers in the prevalent heart disease stratum, but the results might be opposite<br />
for the non-heart disease stratum. Similar results were observed for rs10918594 minor allele carriers.<br />
We observed no effect of NOS1AP genotype on sys<strong>to</strong>lic or dias<strong>to</strong>lic function in non-users of digoxin.<br />
In table 6, the results for an allelic model are displayed (where applicable) stratified on digoxin use and<br />
prevalent heart disease, but none of the results showed a significant association.<br />
142