pH of Polybasic acid buffers
pH of Polybasic acid buffers
pH of Polybasic acid buffers
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>pH</strong> <strong>of</strong> <strong>Polybasic</strong> <strong>acid</strong> <strong>buffers</strong><br />
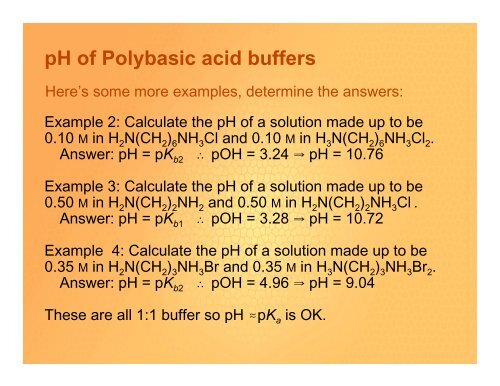
Here’s some more examples, determine the answers:<br />
Example 2: Calculate the <strong>pH</strong> <strong>of</strong> a solution made up to be<br />
0.10 M in H 2 N(CH 2 ) 6 NH 3 Cl and 0.10 M in H 3 N(CH 2 ) 6 NH 3 Cl 2 .<br />
Answer: <strong>pH</strong> = pK b2 ˆ pOH = 3.24 Y <strong>pH</strong> = 10.76<br />
Example 3: Calculate the <strong>pH</strong> <strong>of</strong> a solution made up to be<br />
0.50 M in H 2 N(CH 2 ) 2 NH 2 and 0.50 M in H 2 N(CH 2 ) 2 NH 3 Cl .<br />
Answer: <strong>pH</strong> = pK b1 ˆ pOH = 3.28 Y <strong>pH</strong> = 10.72<br />
Example 4: Calculate the <strong>pH</strong> <strong>of</strong> a solution made up to be<br />
0.35 M in H 2 N(CH 2 ) 3 NH 3 Br and 0.35 M in H 3 N(CH 2 ) 3 NH 3 Br 2 .<br />
Answer: <strong>pH</strong> = pK b2 ˆ pOH = 4.96 Y <strong>pH</strong> = 9.04<br />
These are all 1:1 buffer so <strong>pH</strong> .pK a is OK.