pH of Polybasic acid buffers
pH of Polybasic acid buffers
pH of Polybasic acid buffers
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
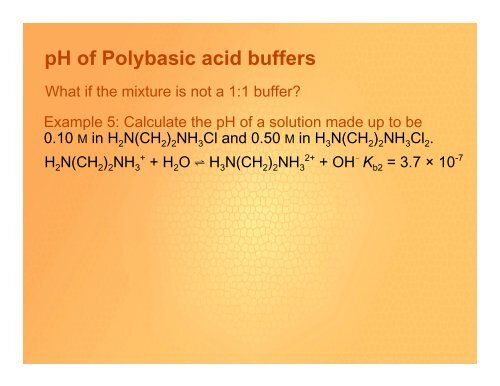
<strong>pH</strong> <strong>of</strong> <strong>Polybasic</strong> <strong>acid</strong> <strong>buffers</strong><br />
What if the mixture is not a 1:1 buffer?<br />
Example 5: Calculate the <strong>pH</strong> <strong>of</strong> a solution made up to be<br />
0.10 M in H 2 N(CH 2 ) 2 NH 3 Cl and 0.50 M in H 3 N(CH 2 ) 2 NH 3 Cl 2 .<br />
H 2 N(CH 2 ) 2 NH 3 + + H 2 O º H 3 N(CH 2 ) 2 NH 3 2+ + OH ! K b2 = 3.7 × 10 -7