pH of Polybasic acid buffers
pH of Polybasic acid buffers
pH of Polybasic acid buffers
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>pH</strong> <strong>of</strong> <strong>Polybasic</strong> <strong>acid</strong> <strong>buffers</strong><br />
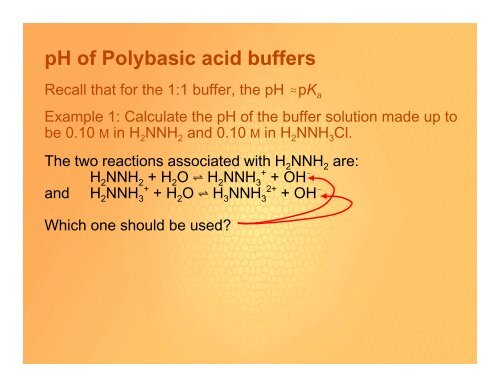
Recall that for the 1:1 buffer, the <strong>pH</strong> .pK a<br />
Example 1: Calculate the <strong>pH</strong> <strong>of</strong> the buffer solution made up to<br />
be 0.10 M in H 2 NNH 2 and 0.10 M in H 2 NNH 3 Cl.<br />
The two reactions associated with H 2 NNH 2 are:<br />
H 2 NNH 2 + H 2 O º H 2 NNH 3 + + OH !<br />
and H 2 NNH 3 + + H 2 O º H 3 NNH 3 2+ + OH !<br />
Which one should be used?