pH of Polybasic acid buffers
pH of Polybasic acid buffers
pH of Polybasic acid buffers
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>pH</strong> <strong>of</strong> <strong>Polybasic</strong> <strong>acid</strong> <strong>buffers</strong><br />
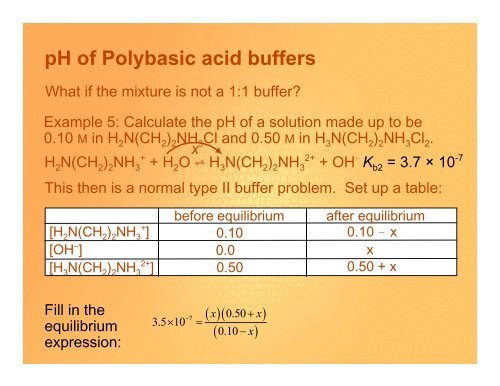
What if the mixture is not a 1:1 buffer?<br />
Example 5: Calculate the <strong>pH</strong> <strong>of</strong> a solution made up to be<br />
0.10 M in H 2 N(CH 2 ) 2 NH 3 Cl and 0.50 M in H 3 N(CH 2 ) 2 NH 3 Cl 2 .<br />
x<br />
H 2 N(CH 2 ) 2 NH + 3 + H 2 O º H 3 N(CH 2 ) 2 NH 2+ 3 + OH ! K b2 = 3.7 × 10 -7<br />
This then is a normal type II buffer problem. Set up a table:<br />
[H 2 N(CH 2 ) 2 NH + 3 ]<br />
[OH – ]<br />
[H 3 N(CH 2 ) 2 NH 2+ 3 ]<br />
before equilibrium after equilibrium<br />
0.10<br />
0.0<br />
0.10 ! x<br />
x<br />
0.50<br />
0.50 + x<br />
Fill in the<br />
equilibrium<br />
expression:<br />
( x)( x)<br />
( 0.10 − x)<br />
− 7<br />
0.50 +<br />
3.5× 10 =