pH of Polybasic acid buffers
pH of Polybasic acid buffers
pH of Polybasic acid buffers
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>pH</strong> <strong>of</strong> <strong>Polybasic</strong> <strong>acid</strong> <strong>buffers</strong><br />
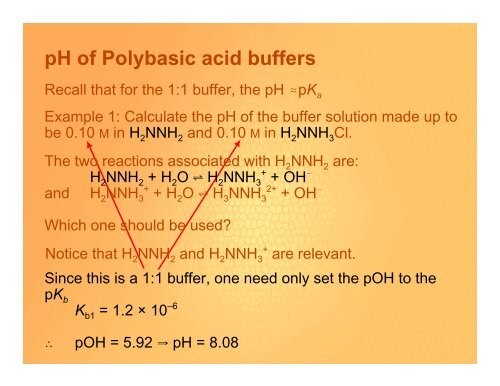
Recall that for the 1:1 buffer, the <strong>pH</strong> .pK a<br />
Example 1: Calculate the <strong>pH</strong> <strong>of</strong> the buffer solution made up to<br />
be 0.10 M in H 2 NNH 2 and 0.10 M in H 2 NNH 3 Cl.<br />
The two reactions associated with H 2 NNH 2 are:<br />
H 2 NNH 2 + H 2 O º H 2 NNH 3 + + OH !<br />
and H 2 NNH 3 + + H 2 O º H 3 NNH 3 2+ + OH !<br />
Which one should be used?<br />
Notice that H 2 NNH 2 and H 2 NNH 3 + are relevant.<br />
Since this is a 1:1 buffer, one need only set the pOH to the<br />
pK b<br />
Kb1 = 1.2 × 10 –6<br />
ˆ pOH = 5.92 Y <strong>pH</strong> = 8.08