The three-dimensional structure of humic substances and soil ...
The three-dimensional structure of humic substances and soil ...
The three-dimensional structure of humic substances and soil ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Fresenius J Anal Chem (I995) 351:62-73 Fresenius' Journal <strong>of</strong><br />
© Springer-Verlag 1995<br />
<strong>The</strong> <strong>three</strong>-<strong>dimensional</strong> <strong>structure</strong> <strong>of</strong> <strong>humic</strong> <strong>substances</strong><br />
<strong>and</strong> <strong>soil</strong> organic matter studied by computational analytical chemistry<br />
H.-R. Schulten<br />
Fachhochschule Fresenius, Department <strong>of</strong> Trace Analysis, Dambachtal 20, D-65193, Wiesbaden, Germany<br />
Received: 8 August 1994/Revised: 29 August 1994/Accepted: 31 August 1994<br />
Abstract. A novel <strong>three</strong>-<strong>dimensional</strong> structural concept<br />
for <strong>humic</strong> <strong>substances</strong> <strong>and</strong> <strong>soil</strong> organic matter (SOM) is<br />
proposed which is based on previously published, com-<br />
prehensive investigations combining geochemical, wet-<br />
chemical, biochemical, spectroscopic, agricultural <strong>and</strong><br />
ecological data with analytical pyrolysis. Direct, tempera-<br />
ture-programmed pyrolysis in the ion-source <strong>of</strong> the mass<br />
spectrometer <strong>and</strong> s<strong>of</strong>t ionization in very high electric<br />
fields (Py-FIMS) <strong>and</strong> Curie-point pyrolysis-gas chroma-<br />
tography/mass spectrometry (Py-GC/MS) were the main<br />
applied thermal methods. Emphasis is laid on molecular<br />
modelling <strong>and</strong> geometry optimization <strong>of</strong> complex, poly-<br />
disperse <strong>structure</strong>s <strong>of</strong> biomacromolecules using modern<br />
PC s<strong>of</strong>tware (HyperChem®). Trapping <strong>and</strong> binding <strong>of</strong><br />
atrazine in an organo-mineral complex is introduced as a<br />
first example <strong>of</strong> simulation experiments for <strong>soil</strong> processes<br />
at atomic level (nanochemistry). Future applications <strong>of</strong><br />
semi-empirical calculations <strong>and</strong> molecular dynamics in<br />
pyrolysis studies are outlined.<br />
Introduction<br />
Since practically all pyrolysis data so far have been ob-<br />
tained <strong>and</strong> published as two-<strong>dimensional</strong> (2 D) plots, it is<br />
<strong>of</strong> interest to illustrate recent progress in commercially<br />
available, relatively low cost s<strong>of</strong>tware <strong>and</strong> personal com-<br />
puter (PC) equipment which allow <strong>three</strong>-<strong>dimensional</strong><br />
(3D) displays <strong>and</strong> computer-assisted design (CAD) <strong>of</strong><br />
chemical <strong>structure</strong>s <strong>and</strong> model reactions. In particular<br />
the possibilities for molecular modelling <strong>and</strong> geometry<br />
optimizations <strong>of</strong> complex macromolecules, which are <strong>of</strong>-<br />
ten the target <strong>of</strong> analytical pyrolysis, virtually open up a<br />
new dimension. This is demonstrated in the following for<br />
<strong>humic</strong> <strong>substances</strong>, one <strong>of</strong> the most complex naturally oc-<br />
curring materials, as an example.<br />
Dedicated to Pr<strong>of</strong>essor Dr. Dieter Klockow on the occasion <strong>of</strong> his 60th<br />
birthday<br />
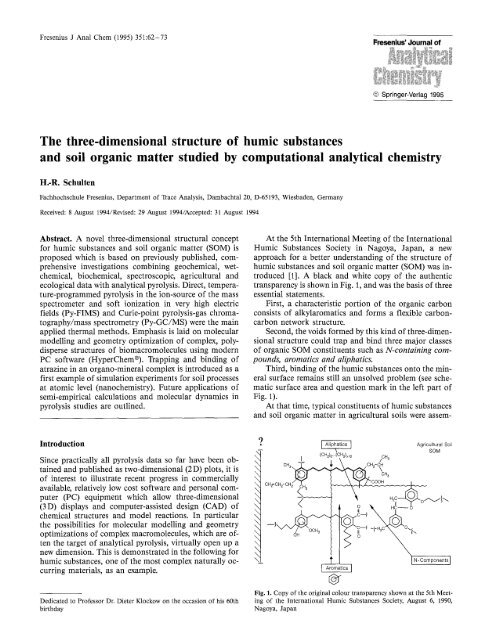
At the 5th International Meeting <strong>of</strong> the International<br />
Humic Substances Society in Nagoya, Japan, a new<br />
approach for a better underst<strong>and</strong>ing <strong>of</strong> the <strong>structure</strong> <strong>of</strong><br />
<strong>humic</strong> <strong>substances</strong> <strong>and</strong> <strong>soil</strong> organic matter (SOM) was in-<br />
troduced [1]. A black <strong>and</strong> white copy <strong>of</strong> the authentic<br />
transparency is shown in Fig. 1, <strong>and</strong> was the basis <strong>of</strong> <strong>three</strong><br />
essential statements.<br />
First, a characteristic portion <strong>of</strong> the organic carbon<br />
consists <strong>of</strong> alkylaromatics <strong>and</strong> forms a flexible carbon-<br />
carbon network <strong>structure</strong>.<br />
Second, the voids formed by this kind <strong>of</strong> <strong>three</strong>-dimen-<br />
sional <strong>structure</strong> could trap <strong>and</strong> bind <strong>three</strong> major classes<br />
<strong>of</strong> organic SOM constituents such as N-containing com-<br />
pounds, aromatics <strong>and</strong> aliphatics.<br />
Third, binding <strong>of</strong> the <strong>humic</strong> <strong>substances</strong> onto the min-<br />
eral surface remains still an unsolved problem (see sche-<br />
matic surface area <strong>and</strong> question mark in the left part <strong>of</strong><br />
Fig. 1).<br />
At that time, typical constituents <strong>of</strong> <strong>humic</strong> <strong>substances</strong><br />
<strong>and</strong> <strong>soil</strong> organic matter in agricultural <strong>soil</strong>s were assem-<br />
~ Agricultural Soil<br />
I . \ / 3<br />
i ~ (CH2)6'"~CH2)>lO OH SOM<br />
CH 3. ~ '~ \ ,CH2-CH<br />
/<br />
] Arom Lic~<br />
@r<br />
Fig. 1. Copy <strong>of</strong> the original co]our transparency shown at the 5th Meet-<br />
ing <strong>of</strong> the International Humic Substances Society, August 6, 1990,<br />
Nagoya, Japan
led for N-containing compounds (Table 1), aromatic<br />
(Table 2) <strong>and</strong> aliphatic compounds (Table 3) which result-<br />
ed from analytical pyrolysis studies using mainly Py-<br />
FIMS <strong>and</strong> Py-GC/MS [1]. More than 170 characteristic,<br />
fundamental <strong>structure</strong>s were identified <strong>and</strong> are listed in<br />
Tables 1 to 3 <strong>and</strong>, considering homologues <strong>and</strong> isomers,<br />
these might well represent > 1000 chemical compounds.<br />
This gave the basis for the proposed carbon-carbon net-<br />
work <strong>structure</strong> <strong>of</strong> <strong>humic</strong> <strong>substances</strong> which has been<br />
visualized with its voids <strong>and</strong> flexible covalent links [2]<br />
<strong>and</strong> clearly demonstrated the complexity <strong>and</strong> variability<br />
<strong>of</strong> the model. <strong>The</strong> latter was particularly emphasized <strong>and</strong><br />
indicated by the % symbols which st<strong>and</strong> for a wide range<br />
Table 1. Nitrogen-containing compounds characteristic for <strong>soil</strong> organic matter in agricultural <strong>and</strong><br />
forest <strong>soil</strong>s (status by Py-FIMS <strong>and</strong> Py-GC/MS, August 6, 1990) [1]. Molecular weights are given<br />
in brackets (m/z)<br />
NH3 ( 17 ) @ CH 3 ~ 0 C--CH3 ~ N CH2-CH20H<br />
HC_=N<br />
1181 (93)<br />
11351 1161)<br />
(27) H3C~-- ~ CHS ~ / C----N<br />
0 H<br />
C~3NH2 ~S/ ~CH ~ 0CH3<br />
(311 (95) 3<br />
~-- CHB ~NH2<br />
~,o %N 2--cH3 113s) OH<br />
H--C~-NH 2 11631<br />
H3C C~.NH 2 H 1951 C~2--CH20H 11711<br />
1591<br />
~ OH 1137) ~CO--CH 3<br />
~ C2Hs ~N'-CH3<br />
H (95) H<br />
(67) H (1731<br />
H N ~ CH3<br />
(68) 2 H CH 3 (175)<br />
u 0 ~C00H<br />
(79)<br />
(113)<br />
--CN<br />
OH<br />
0%C/H<br />
(149) ~/~N'~CH3<br />
H<br />
( 175 )<br />
CH3 ~ CH3 CH2--0<br />
(811 (1171<br />
H N~ 3Hv<br />
+=0<br />
CH3 ~ CH3<br />
• 181) (124) (185)<br />
~OH ~ N N'\0CH3 C12H25-C-N<br />
1152) (195)<br />
H<br />
(55) . k-.
64<br />
Table 2. Aromatic compounds characteristic for <strong>soil</strong> organic matter (see Table 1) [1]<br />
CH=CH 2<br />
H3C0 OCH 3<br />
(78) v\CH3 0H<br />
1118) 1134)<br />
(154)<br />
O%C--H ~J_C~N CH3<br />
H 3 OH<br />
(94) ~ CN2-CH3 (CH2)4-CH3<br />
(104)<br />
CH3 Y "0CH3<br />
H3 OH CH=CH-CHa<br />
OCH 3<br />
1120) 0CH 3<br />
(106) CH 3<br />
CH3_C=CH 2<br />
H 3 CH2-CH3 (CH3)2 OCH 3<br />
1106) OH 1164)<br />
(144) OC--CH 3<br />
0CH3 1146) OCH3<br />
(106) OH OH<br />
~OH ~CH3 (124) ~--~CH2)3-CH 3 ~(164)<br />
1108) (130) 1148) 1166)<br />
CH3 CH 3 - CH=CH2 ~3<br />
OH OH<br />
(116) y "OCH 3<br />
(134) OH<br />
ed as a first draft 2D <strong>structure</strong> [3]. It had the elemental<br />
composition <strong>of</strong> C308H328090N 5 with a molecular mass <strong>of</strong><br />
5 540.027 g tool -1 <strong>and</strong> an elemental analysis <strong>of</strong> 66.8% C,<br />
6.0% H, 26.0°70 O, 1.3% N. Compared to isolated stan-<br />
dard HAs with about 56.3070 C <strong>and</strong> 32.8% O, this pro-<br />
posed HA <strong>structure</strong> is obviously too high in carbon <strong>and</strong><br />
too low in oxygen. However, carbohydrates have been re-<br />
ported to account for about 10% <strong>of</strong> the HA weight <strong>and</strong><br />
a similar value has been suggested for proteinaceous ma-<br />
terials in HA [3]. Thus, it has been assumed that one HA<br />
molecule traps within its voids approximately 10% carbo-<br />
hydrates <strong>and</strong> 10% proteinaceous materials. <strong>The</strong> resulting<br />
HA complex has then an elemental composition <strong>of</strong><br />
C342H3880124N12 with a molecular mass <strong>of</strong> 6650.912g<br />
mo1-1 <strong>and</strong> an elemental analysis <strong>of</strong> 61.8% C, 5.9% H,<br />
_CH3 (CHe)s-CH3<br />
(152) 1176)<br />
29.8% O, 2.5% N. If more carbohydrates <strong>and</strong>/or pro-<br />
teinaceous materials are added, the C content decreases<br />
<strong>and</strong> the O content increases.<br />
Thus, for a stereochemical underst<strong>and</strong>ing <strong>of</strong> the<br />
<strong>structure</strong> <strong>of</strong> <strong>humic</strong> <strong>substances</strong> <strong>and</strong> their reactions with<br />
biological <strong>and</strong> anthropogenic compounds <strong>three</strong> main,<br />
urgent questions emerged:<br />
1. What is a fast, reliable <strong>and</strong> versatile method to convert<br />
the 2D <strong>structure</strong> into a 3D <strong>structure</strong>?<br />
2. Is it possible to optimize the geometry <strong>of</strong> the chemical<br />
3 D <strong>structure</strong> <strong>and</strong> to determine <strong>and</strong> minimize its en-<br />
ergy?<br />
3. How can chemical reactions <strong>of</strong> such complex biomac-
Table 2 (continued)<br />
o<br />
CH2-CH2-C~H<br />
0H<br />
CH2-CH:-CHO<br />
H3C0 OCH 3<br />
OCH3 OH<br />
(210)<br />
CH=CH2 (180) 22--(CH2) s-CH3<br />
H3CO~0CH 3 ~ (218)<br />
OKcI{2__CH3(180) ~ ~CH2) 7-cn3<br />
/ ~ 0H (220)<br />
H3CO OCH 3<br />
OH ~H2--( CH2 ) 9-CH3<br />
1182)<br />
©<br />
(232)<br />
CH2--( CH 2 ) io-CH3<br />
OH OK (186) ~--~H3<br />
--( CH 2 ) ~-CH3<br />
(246 )<br />
(1901 ~ -(CH2) II--CH3<br />
H O ~ 12601<br />
1194) ~C6H14<br />
CH2-CHO<br />
TC6HI4<br />
H3C0 0CH 3 (264)<br />
OH<br />
(196) ~--(CH2) 12-CH3<br />
.~--( CH2 ) 7 -CH3<br />
(204)<br />
(274)<br />
¢<br />
CH2--(CH2) 13-CH3<br />
12881<br />
-4CH 2114-CH3<br />
(302)<br />
-qCH 2 ) 15-CH3<br />
1316)<br />
-~CH 2 ) 16-CH3<br />
(330)<br />
romolecules be simulated at atomic <strong>and</strong> molecular<br />
level?<br />
Experimental<br />
Pyrolysis-fieM ionization mass spectrometry (Py-FIMS)<br />
For temperature-resolved Py-FIMS, about 100~tg <strong>of</strong><br />
<strong>humic</strong> <strong>substances</strong> such as <strong>humic</strong> acid (HA), fulvic acid<br />
(FA), humin or 5 mg <strong>of</strong> whole <strong>soil</strong> samples, respectively,<br />
were thermally degraded in the ion source <strong>of</strong> a MAT 731<br />
(Finnigan, 28127 Bremen, Germany) modified high-per-<br />
formance (AMD Intectra GmbH, 27243 Harpstedt, Ger-<br />
many) mass spectrometer. <strong>The</strong> samples were weighed be-<br />
Table 3. Aliphatic compounds characteristic for <strong>soil</strong> organic matter<br />
(see Table i) [11<br />
CH2=CHCHO<br />
CH3-C0--CH 3<br />
CH3-C00H<br />
CHa--CO--CHO<br />
CH2-C--CO--CH 3<br />
CN3-CH--CH--CH--CH--CH 3<br />
3 H C ~ CH3<br />
65<br />
(56)<br />
(58)<br />
(601<br />
(72)<br />
(84)<br />
(96)<br />
0<br />
(i12)<br />
Qo 1114)<br />
3HC-~ OH<br />
11281<br />
0 0 0<br />
II II II<br />
CH3-C--CH2-C--CH2-C--CH 3<br />
11421<br />
~H3 ?H3 ~H3<br />
CH3-CH--(CH2) 3--CH--(CH2) 3-CH--CH2=CH--CH2 (224)<br />
CH CH 3 CH 3<br />
3 i i<br />
CH3--CH--~CH2)3--CH---~CH 2 3~CH~--~CH2 2-C~3<br />
CH3--~ CH 2 )IT"CH20H<br />
CH 3 CH 3 CH 3 CH 3<br />
I I I<br />
CH3-CK--~CH 2 3-CN--CH 2 3-CH--CN 2 3-C=CH2<br />
n=2-30 series<br />
CH3-~ CH 2 )~--CH3<br />
n=i-28 series<br />
CH2=CH--( CH 2 )~--CH 3<br />
CH3--( CH 2 )~4 COOH<br />
(226)<br />
12281<br />
(266)<br />
(58-450)<br />
(56-4341<br />
(256)<br />
14101<br />
fore <strong>and</strong> after Py-FIMS (error + 0.01 mg) to determine the<br />
pyrolysis residue <strong>and</strong> the produced volatile matter. <strong>The</strong><br />
heatable/coolable direct introduction system with elec-<br />
tronic temperature-programming, adjusted at the + 8 kV<br />
potential <strong>of</strong> the ion source <strong>and</strong> the field ionization emit-<br />
ter, was used. <strong>The</strong> slotted cathode plate serving as count-<br />
er electrode was on - 6 kV potential. Thus, at 2 mm dis-<br />
tance between the emitter tips <strong>and</strong> the cathode, in total a<br />
potential difference <strong>of</strong> 14 kV is applied resulting in an ex-<br />
tremely high electric field strength which is the essential<br />
basis for s<strong>of</strong>t ionization. All samples were heated in high<br />
vacuum (1.3 × 10 -4 Pa) from 323 K to 973 K at a heating<br />
rate <strong>of</strong> approximately 0.5Ks -1. About 60magnetic
66<br />
(CH3)o-3<br />
(CHs)o-2<br />
0 ~<br />
@O ~<br />
(CH3) 0-5 @<br />
OH<br />
H O~'.1<br />
oH°'f -° oH°-.-r "°H ,o,<br />
OH OH<br />
scans were recorded for the mass range 16 to 1000<br />
Daltons. In general, at least <strong>three</strong> replicates were per-<br />
formed for each sample. <strong>The</strong> total ion intensities (TII) <strong>of</strong><br />
the single spectra were normalized to 1 mg sample weight,<br />
averaged for replicate runs, <strong>and</strong> plotted versus the<br />
pyrolysis temperature, resulting in Py-FIMS thermo-<br />
grams. For the selection <strong>of</strong> biomarkers <strong>and</strong> quantitative<br />
evaluations, in particular <strong>of</strong> whole <strong>soil</strong>s <strong>and</strong> <strong>soil</strong> particle-<br />
size fractions, detailed descriptions <strong>of</strong> the method have<br />
been given recently [6-9].<br />
HO<br />
OH<br />
Curie-point pyrolysis-gas chromatography~mass<br />
spectrometry (Py-GC/MS)<br />
<strong>The</strong> <strong>humic</strong> <strong>substances</strong> <strong>and</strong> <strong>soil</strong>s were pyrolyzed in a type<br />
0316 Curie-point pyrolyzer (Fischer, 53340 Meckenheim,<br />
Germany). <strong>The</strong> samples were not pretreated except drying<br />
<strong>and</strong> milling. <strong>The</strong> final pyrolysis temperatures (Tf) em-<br />
ployed were 573 K, 773 K <strong>and</strong> 973 K, respectively. <strong>The</strong> to-<br />
tal heating time (THT) was varied between 3 <strong>and</strong> 9.9 s.<br />
Following split injection (split ratio 1:3; flow rate<br />
1 ml 20 s -1) the pyrolysis products were separated on a<br />
gas chromatograph (Varian 3700, 64289 Darmstadt, Ger-<br />
many), equipped with a 30 m capillary column (DB5),<br />
coated with 0.25 gm film thickness <strong>and</strong> an inner diameter<br />
<strong>of</strong> 0.32 mm. <strong>The</strong> starting temperature for the gas chro-<br />
matographic temperature program was 313 K, <strong>and</strong> the<br />
end temperature was 523 K, with a heating rate <strong>of</strong><br />
10 K min -1. <strong>The</strong> gas chromatograph was connected to a<br />
nitrogen-selective, thermosensitive detector (TSD) <strong>and</strong> a<br />
double-focusing Finnigan MAT 212 mass spectrometer.<br />
Conditions for mass spectrometric detection in the elec-<br />
tron ionization mode were +3 kV accelerating voltage,<br />
70eV electron energy, 2.2kV multiplier voltage, 1.1 s/<br />
OH<br />
HO<br />
HO<br />
(CH3)0-2<br />
/.(N ~.,.,2 (CH3)0-2<br />
H<br />
C=N<br />
OH<br />
~0/ ~C_. ?<br />
O<br />
HO =N<br />
[~OH /CH2OH Fig. 2. Drawing <strong>of</strong> the proposed<br />
<strong>humic</strong> acid <strong>structure</strong> using Indian<br />
ink <strong>and</strong> a chemical stencil as published<br />
in Ref. [3]<br />
mass decade scan speed <strong>and</strong> a recorded mass range be-<br />
tween m/z 50 <strong>and</strong> m/z 500. A detailed description <strong>of</strong> the<br />
principle, potential <strong>and</strong> limitations <strong>of</strong> Py-GC/MS <strong>of</strong><br />
<strong>humic</strong> fractions <strong>and</strong> <strong>soil</strong>s has been given [10]. Further-<br />
more, studies <strong>of</strong> organic nitrogen-containing compounds<br />
in <strong>soil</strong>s using the TSD detector were reported [11] which<br />
exp<strong>and</strong>ed the knowledge on the <strong>structure</strong> <strong>of</strong> 'unknown'<br />
<strong>soil</strong> nitrogen beyond the status reported in Tables 1- 3.<br />
Structural modelling <strong>and</strong> geometry optimization<br />
For all described 2D- <strong>and</strong> 3 D-work, model construction,<br />
chemical interaction studies <strong>and</strong> semi-empirical calcula-<br />
tions, the HyperChem ® s<strong>of</strong>tware (release 2 for windows)<br />
[12] was used which is assisted by a comprehensive manu-<br />
al. In the present text some main s<strong>of</strong>tware comm<strong>and</strong>s are<br />
indicated in brackets (in italics). <strong>The</strong> program is supplied<br />
by Computer Aided Animation (CAA) GmbH, Kenner-<br />
weg 15, 72622 Ntirtingen, Germany.<br />
<strong>The</strong> employed PC consisted <strong>of</strong> the Escom local-bus<br />
tower 486DX2/66, VLB 34 in combination with 8 MB<br />
memory, Escom 17" colour monitor with AVGA VL<br />
bus/1MB graphic card, Samsung 250 MB disk, 250 MB<br />
powerstreamer, <strong>and</strong> peripheric hardware plus utility pro-<br />
grams (Escom <strong>of</strong>fice, Rheinstr. 41, D-65185 Wiesbaden,<br />
Germany).<br />
Results <strong>and</strong> discussion<br />
Building molecules<br />
Building a 3 D model <strong>of</strong> the 2D <strong>humic</strong> acid <strong>structure</strong><br />
(Fig. 2) is performed in five steps using the HyperChem ®<br />
s<strong>of</strong>tware.
First, a preliminary 2 D <strong>structure</strong> <strong>of</strong> the C-C skeleton<br />
is drawn by h<strong>and</strong> into the workspace using the drawing<br />
tool <strong>and</strong> is far from perfect as shown in Fig. 3. In addi-<br />
tion to carbon, the elements oxygen <strong>and</strong> nitrogen are in-<br />
cluded (Default Element). In this manner 308 carbon-,<br />
90 oxygen- <strong>and</strong> 5 nitrogen-atoms (403 atoms in total) are<br />
positioned.<br />
Second, hydrogen is attached with accurate bond<br />
lengths <strong>and</strong> angles to the preliminary <strong>structure</strong> using the<br />
s<strong>of</strong>tware (Add Hydrogen) as shown in Fig. 4. Whereas in<br />
the original publication the 2 D HA subunit <strong>structure</strong> [2]<br />
was incomplete <strong>and</strong> was supposed to have a wide variety<br />
<strong>of</strong> different bonding sequences (see :k-- symbols), the mol-<br />
ecule in Fig. 4 represents a defined, intact HA monomer<br />
(738 atoms). Thus, in order to obtain a complete chemi-<br />
cal compound, it had to be supplemented by 7 hydrogen<br />
atoms. <strong>The</strong> corresponding elemental composition then is<br />
C308H335090N 5 with a molecular mass <strong>of</strong> 5547.004g<br />
mol -~ <strong>and</strong> an elemental analysis <strong>of</strong> 66.69% C, 6.09% H,<br />
25.96% O, <strong>and</strong> 1.26% N.<br />
Third, building the 3 D <strong>structure</strong> <strong>of</strong> the selected HA<br />
molecule (Model BuiM) with accurate bond distances<br />
<strong>and</strong> bond angles, torsion angles, van der Waals forces,<br />
<strong>and</strong> hydrogen bonds, is illustrated in Fig. 5. This prelimi-<br />
nary, energetically still unoptimized <strong>structure</strong> shows<br />
clearly the atomic distances <strong>and</strong> angles (Sticks) <strong>and</strong> gives<br />
a first <strong>three</strong>-<strong>dimensional</strong> <strong>structure</strong> information. Even this<br />
rough display indicates already the importance <strong>of</strong><br />
aliphatic chains as link between the aromatic moieties<br />
<strong>and</strong> prerequisite for the formation <strong>of</strong> gaps <strong>and</strong> voids in<br />
the HA <strong>structure</strong>.<br />
Fourth, the (Sticks) <strong>structure</strong> in Fig. 5 is converted in-<br />
to the 3 D <strong>structure</strong> (Disks, Perspective) shown in Fig. 6<br />
which better reflects the space requirements <strong>of</strong> the 738 at-<br />
oms. <strong>The</strong> white disks are hydrogen atoms, black disks ni-<br />
trogen, the brighter shaded disks are carbons <strong>and</strong> the<br />
darker ones oxygen. It is evident that even more complex<br />
<strong>structure</strong>s such as naturally occurring organomineral<br />
complexes or trapping <strong>of</strong> biological <strong>and</strong> anthropogenic<br />
<strong>substances</strong> in SOM will be difficult to evaluate in these<br />
Fig. 3. H<strong>and</strong>drawn two-<strong>dimensional</strong> version (2D) <strong>of</strong> the <strong>humic</strong> acid<br />
draft <strong>structure</strong> using the drawing tool in the HyperChem ® workspace<br />
-2<br />
Fig. 4. Hydrogen attachment to the h<strong>and</strong>drawn <strong>humic</strong> acid draft struc-<br />
ture performed by HyperChem ® (Add Hydrogen)<br />
black <strong>and</strong> white plots. As will be illustrated in the follow-<br />
ing fifth step, colourplots can be <strong>of</strong> great help <strong>and</strong> allow<br />
a distinct view <strong>and</strong> facilitated evaluation <strong>of</strong> the complex,<br />
large macromolecules.<br />
Chemical calculations<br />
After building the molecule from scratch (Fig. 3), the<br />
atomic coordinates in the s<strong>of</strong>tware files allow a wide<br />
range <strong>of</strong> quantitative evaluations. Measuring structural<br />
properties includes the determination <strong>of</strong> bond distances,<br />
bond angles, torsion angles, non-bonded distances, <strong>and</strong><br />
hydrogen bonds. For minimizing the total energy <strong>of</strong> the<br />
molecular system <strong>and</strong> thus increasing the stability <strong>of</strong> its<br />
conformation, step-by-step instructions for performing<br />
single point calculations, geometry optimization, <strong>and</strong><br />
molecular dynamics are given by HyperChem ®.<br />
When one cycle <strong>of</strong> a single-point calculation <strong>of</strong> the<br />
HA 3 D <strong>structure</strong> in Fig. 6 was performed using the algo-<br />
rithm <strong>of</strong> Polak-Ribiere (conjugate gradient), the total en-<br />
Fig. 5. Three-<strong>dimensional</strong> <strong>structure</strong> (3D) <strong>of</strong> the draft HA <strong>structure</strong><br />
shown in Fig. 4 using HyperChem ® for molecular modelling (Build<br />
Model). Display <strong>of</strong> the <strong>structure</strong> in the sticks mode (Rendering; Sticks)<br />
67
68<br />
ergy <strong>of</strong> the molecular system was calculated with<br />
6993.92 kJ 0.1 nm -1 mo1-1 <strong>and</strong> a gradient (derivative <strong>of</strong><br />
the energy with respect to all Cartesian coordinates) <strong>of</strong><br />
91.16 kJ 0.1 nm -1 mol -I. <strong>The</strong> termination conditions<br />
for this calculation were 83.80 kJ nm- 1 mol - 1 <strong>and</strong> 11070<br />
cycles maximum. After approximately 4700 calculation<br />
Fig. 6. First approximation (1 cycle, 3 points, convergence limit <strong>of</strong><br />
83.80 kJ 0.1 nm -1 mol ~) for the geometrical optimization <strong>of</strong> the HA<br />
<strong>structure</strong> (II) given in Fig. 5 (Compute; Single Point; Geometry Optimi-<br />
zation) by semi-empirical calculations. Display <strong>of</strong> the <strong>structure</strong> in the<br />
disks mode (Rendering; Disks)<br />
~ J<br />
Fig. 7. Geometry optimization<br />
<strong>and</strong> energy minimization <strong>of</strong> the<br />
3 D <strong>structure</strong> <strong>of</strong> HA (II, Fig. 6)<br />
after 4700 calculation cycles <strong>and</strong><br />
a resulting total energy <strong>of</strong><br />
710.70 kJ nm -1 mo1-1 <strong>and</strong> a<br />
convergence gradient <strong>of</strong><br />
0.037 kJ nm- 1 mol- 1<br />
cycles with a convergence limit <strong>of</strong> 0.83 kJ 0.1 nm -1<br />
tool-l, the energy minimization leads to a molecular en-<br />
ergy <strong>of</strong> 752.57kJ 0.1 nm -1 tool -1 with a gradient <strong>of</strong><br />
0.71 kJ 0.1 nm -1 tool -1. As an illustration <strong>of</strong> the energy<br />
minimization process in Fig. 7 the corresponding HA<br />
<strong>structure</strong> is displayed. Rotation <strong>of</strong> the energy-minimized
HA 3 D version demonstrates the flexible network with<br />
voids <strong>and</strong> hollows which <strong>of</strong>fer binding <strong>and</strong> trapping <strong>of</strong><br />
biological <strong>and</strong> anthropogenic molecules [2]. Further-<br />
more, long-continued calculation periods (> 6 days) give<br />
energies in the order <strong>of</strong> 608 kJ 0.1 nm-1 mol-1 <strong>and</strong> gra-<br />
dients
70<br />
Fig. 9. Result <strong>of</strong> atrazine transport into the 3 D structural model <strong>of</strong> an organo-mineral complex in <strong>soil</strong> with atrazine trapped in the voids (V)<br />
the atrazine molecule moves with decreasing energy <strong>and</strong><br />
increasing conformational stability step-by-step more<br />
into the available space <strong>of</strong> the void. Finally, arriving with<br />
the geometry optimization <strong>and</strong> energy minimization<br />
close to a total energy <strong>of</strong> 10008.21 kJ nm -1 mo1-1 <strong>and</strong> a<br />
gradient <strong>of</strong> 0.37 kJ nm-1 mol-t, the atrazine molecule is<br />
immobilized in the void by formation <strong>of</strong> a hydrogen bond<br />
from H (21, molecule I) to a HA carboxyl group <strong>of</strong> II (O<br />
(606, molecule II)) which is an integral part <strong>of</strong> the<br />
organo-mineral particle V now. Figure 10c shows the<br />
space requirements <strong>of</strong> the atrazine molecule in the hollow<br />
space, but also, may be more important, how much space<br />
is left for chemical decomposition reactions.<br />
Nanochemistry<br />
For the biological <strong>and</strong> chemical reactivity <strong>of</strong> the trapped<br />
anthropogenic compound it is highly interesting to deter-<br />
mine exactly the distances between I <strong>and</strong> the surrounding<br />
molecular complex <strong>of</strong> the HA II <strong>and</strong> the silica matrix IH.<br />
As derived from Fig. 10a, the numbered atoms <strong>and</strong><br />
Fig. 10b the atoms labelled by element symbols, some
~749 ~54<br />
Fig. 10a-e. <strong>The</strong> enlarged section <strong>of</strong> a void in an organo-mineral com-<br />
plex (V; geometry <strong>and</strong> energy optimization: bonds <strong>and</strong> angles, torsions,<br />
van de Waals forces, hydrogen bridges) is shown in which atrazine is<br />
trapped <strong>and</strong> fixed via a distinct hydrogen bond <strong>and</strong> SOM is bound to<br />
the mineral matrix via hydrogen bonding in addition to <strong>three</strong> covalent<br />
bonds, a Section (Sticks, Atoms, Number) is shown with the displayed<br />
selected distances between atrazine I <strong>and</strong> II <strong>and</strong> III are as<br />
follows:<br />
Starting from C1 (i, I) (atom 1, molecule I) the dis-<br />
tances to H (256, III) are 0.7947 nm (HyperChem ® out-<br />
put 7.947477 Angstrom), to H (255, III) 1.0466 nm, to O<br />
(225, III) 0.3737 nm, O (187, III) 0.3431 nm, O (100, III)<br />
0.8717 nm <strong>and</strong> to the hydrogens <strong>of</strong> the long aliphatic<br />
chain (see lower part <strong>of</strong> V in Fig. 9) H (1004, II)<br />
0.6051 nm <strong>and</strong> H (1011, II) 0.8632 nm. From H (3, I) to<br />
H (950, II) a distance <strong>of</strong> 0.3074 nm, to H (996, II) <strong>of</strong><br />
0.2160 nm <strong>and</strong> to H (1004, II) <strong>of</strong> 0.4177 nm was measured<br />
for the lower part <strong>of</strong> the void. In the upper part, for in-<br />
stance between H (17, I) <strong>and</strong> H (902, II) 0.3074 nm, H<br />
(749, II) 0.4468 nm, H (745, II) 0.6537 nm, C (400, II)<br />
0.7601 rim, O (577, II) 0.4885 nm were obtained. Despite<br />
the apparent proximity between H (19, I) <strong>and</strong> H (251, III)<br />
a distance <strong>of</strong> 1.1614 nm was found <strong>and</strong> clearly demon-<br />
strated the necessity <strong>of</strong> exactly calculated coordinate<br />
systems. Moreover, the distance between H (21, I) <strong>and</strong><br />
O (606, II) was <strong>of</strong> interest, because it narrowed with<br />
progressing geometry optimization from >1rim to<br />
0.2221 nm generating a hydrogen bond which immobi-<br />
lized the atrazine molecule in the HA void. <strong>The</strong> more the<br />
total energy content <strong>of</strong> the organo-mineral complex V is<br />
/<br />
1<br />
6<br />
4<br />
6~4 "~<br />
71<br />
atoms labelled by numbers; b Section (Sticks, Atoms, Symbols) is<br />
shown with atoms labelled by element symbols; c Section (Rendering;<br />
Disks, Perspective) is shown as colour plot illustrating the space re-<br />
quirements <strong>of</strong> the pesticide <strong>and</strong> the available <strong>three</strong>-<strong>dimensional</strong> trap-<br />
ping space<br />
lowered, the more shrinking <strong>of</strong> the hollow space <strong>and</strong> con-<br />
sequently stronger bonding <strong>of</strong> the atrazine is observed. In<br />
summary, one can assume that without operator interfer-<br />
ence energy minimization simulates aging processes.<br />
<strong>The</strong> results <strong>of</strong> the <strong>three</strong>-<strong>dimensional</strong> <strong>structure</strong> <strong>and</strong> dis-<br />
tance measurements furthermore allow to evaluate the<br />
space between the trapped molecule <strong>and</strong> its host, the<br />
organo-mineral complex. In particular the space filling <strong>of</strong><br />
trapped biological or anthropogenic <strong>substances</strong> is sup-<br />
posed to have an essential influence on their presumable<br />
fate in SOM. Clearly air (N2, O2) <strong>and</strong> water (HzO) <strong>and</strong><br />
small organic acids (CH3COOH) can invade the gaps be-<br />
tween the pesticide <strong>and</strong> organic or inorganic <strong>soil</strong> surfaces.<br />
Thus, non-metabolic decomposition processes are easily<br />
possible at this stage. Metabolic processes, however, can<br />
be excluded as microorganisms such as bacteria (> 200 to<br />
1000 nm) <strong>and</strong> fungi (> 10000 nm) have no access into the<br />
described voids <strong>and</strong> even enzymes are too large to react<br />
with the trapped materials when the front <strong>and</strong> back side<br />
<strong>of</strong> the void is closed by recalcitrant SOM or inorganic<br />
<strong>substances</strong>. <strong>The</strong> latter is much more likely, as the organic<br />
matter in agricultural <strong>soil</strong>s is only around 3°70. Thus, in<br />
nature the inorganic matrix represented here by the<br />
hypothetic model III should be larger by at least a factor<br />
<strong>of</strong> 30.
72<br />
Fig. 10b<br />
\<br />
Fig. 10c
It is expected that computational chemistry in combi-<br />
nation with analytical pyrolysis (<strong>and</strong> analytical chemistry<br />
in general) will play an important role in future studies<br />
not only in environmental studies [14] <strong>and</strong> ecology [15]<br />
but also in <strong>soil</strong> science [16] <strong>and</strong> agriculture as described<br />
recently [13, 17].<br />
Conclusions<br />
(1) <strong>The</strong> molecular-chemical <strong>structure</strong> <strong>of</strong> <strong>soil</strong> organic mat-<br />
ter is mainly determined by the inorganic <strong>soil</strong> matrix, the<br />
climate, plant precursors, <strong>and</strong> the dynamics <strong>and</strong> specific<br />
activity <strong>of</strong> biomass.<br />
(2) <strong>The</strong> resulting <strong>three</strong>-<strong>dimensional</strong>, organo-mineral<br />
complexes <strong>of</strong> decomposition products, biologically syn-<br />
thesized materials <strong>and</strong> <strong>soil</strong> mineral particles can be ana-<br />
lyzed indirectly by Py-GC/MS <strong>and</strong> directly by in-source<br />
Py-MS.<br />
(3) Analytical pyrolysis <strong>of</strong> <strong>humic</strong> <strong>substances</strong> <strong>and</strong><br />
whole <strong>soil</strong>s yields a vast variety <strong>of</strong> pyrolysis products.<br />
<strong>The</strong>ir intra- <strong>and</strong> intermolecular chemical properties are<br />
only understood when fast, reliable <strong>and</strong> powerful com-<br />
puter-assisted data h<strong>and</strong>ling <strong>and</strong> evaluating allows the<br />
chemistry to evolve in the <strong>three</strong> dimensions <strong>of</strong> space un-<br />
der the precise structural parameters such bond lengths,<br />
angles, <strong>and</strong> torsions, van der Waals forces, hydrogen<br />
bridges, etc.<br />
(4) To reassemble the identified chemical subunits to<br />
a hypothetic <strong>structure</strong> <strong>of</strong> <strong>soil</strong> organic matter that reflects<br />
as closely as possible the genuine status, above all con-<br />
structive interdisciplinary work <strong>and</strong> collaboration is<br />
needed.<br />
Acknowledgements. <strong>The</strong> author is grateful for the financial support by<br />
the Deutsche Forschungsgemeinschaft (projects Schu 416/3; Schu 416/<br />
18-1) <strong>and</strong> the Ministry <strong>of</strong> Science <strong>and</strong> Technology, Bonn-Bad Godes-<br />
berg, <strong>and</strong> the Umweltbundesamt, Berlin, Germany. Thanks are due to<br />
R. Hempfling, R. Mt~ller, C. Sorge <strong>and</strong> H. Wilken (Fresenius Group)<br />
<strong>and</strong> P. Leinweber, University <strong>of</strong> Osnabrack for their help <strong>and</strong> coopera-<br />
73<br />
tion. To work with the "pioneer <strong>of</strong> <strong>humic</strong> <strong>substances</strong>" Dr. Morris<br />
Schnitzer, Agriculture Canada, Ottawa, has been <strong>and</strong> is a unique occa-<br />
sion which gives its merits <strong>and</strong> rewards by itself.<br />
References<br />
1. Schulten H-R (1990) A contribution to solving the puzzle <strong>of</strong> the<br />
chemical <strong>structure</strong> <strong>of</strong> <strong>humic</strong> <strong>substances</strong>: pyrolysis-s<strong>of</strong>t ionization<br />
mass spectrometry. 5th International Meeting, International Humic<br />
Substances Society, August 6-10, Nagoya, Japan<br />
2. Schulten H-R, Plage B, Schnitzer M (1991) Naturwissenschaften<br />
78:311<br />
3. Schulten H-R, Schnitzer M (1993) Naturwissenschaften 80:29<br />
4. Schulten H-R (1994) A chemical <strong>structure</strong> for <strong>humic</strong> acid. Pyroly-<br />
sis-gas chromatography/mass spectrometry <strong>and</strong> pyrolysis-s<strong>of</strong>t ion-<br />
ization mass spectrometry evidence. In: Senesi N, Miano TM (eds)<br />
Humic <strong>substances</strong> in the global environment <strong>and</strong> implications on<br />
human health. Elsevier, Amsterdam, pp 43- 56<br />
5. Schnitzer M (1994) A chemical <strong>structure</strong> for <strong>humic</strong> acid. Chemical,<br />
~3C NMR, colloid chemical <strong>and</strong> electron microscopic evidence. In:<br />
Senesi N, Miano TM (eds) Humic <strong>substances</strong> in the global environ-<br />
ment <strong>and</strong> implications on human health. Elsevier, Amsterdam, pp<br />
57 - 69<br />
6. Schulten H-R (1993) J Anal Appl Pyrolysis 25:97 (<strong>and</strong> references<br />
cited therein)<br />
7. Schulten H-R, Leinweber P, Sorge C (1993) J Soil Sci 44:677<br />
8. Leinweber P, Schulten H-R, KOrschens M (1994) Plant Soil 160:225<br />
9. Sorge C, Schnitzer M, Leinweber P, Schulten H-R (i994) Soil Sci<br />
158:189<br />
10. Schulten H-R, Schnitzer M (1992) Soil Sci 153:205<br />
11. Schulten H-R, Sorge C, Schnitzer M (1995) Biol Fertil Soil (in<br />
press)<br />
12. HyperChem ® Autodesk Inc, 2320 Marinship Way, Sausalito, CA<br />
94965 USA<br />
13. Schulten H-R (1995) J Anal Appl Pyrolysis (in press)<br />
14. Khan SU, Schnitzer M, Schulten H-R (i993) J Agr Food Chem<br />
41:1143<br />
15. Simmleit N, Schulten H-R (1989) J Anal Appl Pyrolysis 15:3<br />
16. Schulten H-R (1995) Direct pyrolysis - mass spectrometry <strong>of</strong> <strong>soil</strong>s:<br />
a novel tool in agriculture, ecology, forestry <strong>and</strong> <strong>soil</strong> science. In:<br />
Yamasaki S, Boutton TW (eds) Mass spectrometry <strong>of</strong> s<strong>of</strong>ts. Dekker,<br />
New York (in press)<br />
17. Leinweber P, Schulten H-R (1995) J Anal Pyrolysis (in press)





![Legislativa - Označování potravin [režim kompatibility]](https://img.yumpu.com/15533670/1/190x135/legislativa-oznacovani-potravin-rezim-kompatibility.jpg?quality=85)







