The three-dimensional structure of humic substances and soil ...
The three-dimensional structure of humic substances and soil ...
The three-dimensional structure of humic substances and soil ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Table 2 (continued)<br />
o<br />
CH2-CH2-C~H<br />
0H<br />
CH2-CH:-CHO<br />
H3C0 OCH 3<br />
OCH3 OH<br />
(210)<br />
CH=CH2 (180) 22--(CH2) s-CH3<br />
H3CO~0CH 3 ~ (218)<br />
OKcI{2__CH3(180) ~ ~CH2) 7-cn3<br />
/ ~ 0H (220)<br />
H3CO OCH 3<br />
OH ~H2--( CH2 ) 9-CH3<br />
1182)<br />
©<br />
(232)<br />
CH2--( CH 2 ) io-CH3<br />
OH OK (186) ~--~H3<br />
--( CH 2 ) ~-CH3<br />
(246 )<br />
(1901 ~ -(CH2) II--CH3<br />
H O ~ 12601<br />
1194) ~C6H14<br />
CH2-CHO<br />
TC6HI4<br />
H3C0 0CH 3 (264)<br />
OH<br />
(196) ~--(CH2) 12-CH3<br />
.~--( CH2 ) 7 -CH3<br />
(204)<br />
(274)<br />
¢<br />
CH2--(CH2) 13-CH3<br />
12881<br />
-4CH 2114-CH3<br />
(302)<br />
-qCH 2 ) 15-CH3<br />
1316)<br />
-~CH 2 ) 16-CH3<br />
(330)<br />
romolecules be simulated at atomic <strong>and</strong> molecular<br />
level?<br />
Experimental<br />
Pyrolysis-fieM ionization mass spectrometry (Py-FIMS)<br />
For temperature-resolved Py-FIMS, about 100~tg <strong>of</strong><br />
<strong>humic</strong> <strong>substances</strong> such as <strong>humic</strong> acid (HA), fulvic acid<br />
(FA), humin or 5 mg <strong>of</strong> whole <strong>soil</strong> samples, respectively,<br />
were thermally degraded in the ion source <strong>of</strong> a MAT 731<br />
(Finnigan, 28127 Bremen, Germany) modified high-per-<br />
formance (AMD Intectra GmbH, 27243 Harpstedt, Ger-<br />
many) mass spectrometer. <strong>The</strong> samples were weighed be-<br />
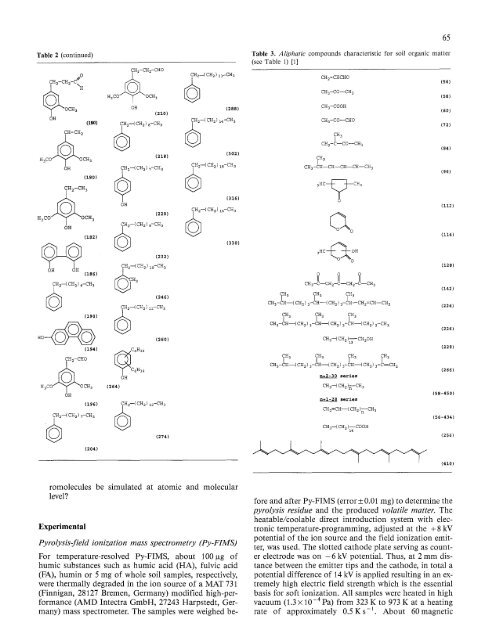
Table 3. Aliphatic compounds characteristic for <strong>soil</strong> organic matter<br />
(see Table i) [11<br />
CH2=CHCHO<br />
CH3-C0--CH 3<br />
CH3-C00H<br />
CHa--CO--CHO<br />
CH2-C--CO--CH 3<br />
CN3-CH--CH--CH--CH--CH 3<br />
3 H C ~ CH3<br />
65<br />
(56)<br />
(58)<br />
(601<br />
(72)<br />
(84)<br />
(96)<br />
0<br />
(i12)<br />
Qo 1114)<br />
3HC-~ OH<br />
11281<br />
0 0 0<br />
II II II<br />
CH3-C--CH2-C--CH2-C--CH 3<br />
11421<br />
~H3 ?H3 ~H3<br />
CH3-CH--(CH2) 3--CH--(CH2) 3-CH--CH2=CH--CH2 (224)<br />
CH CH 3 CH 3<br />
3 i i<br />
CH3--CH--~CH2)3--CH---~CH 2 3~CH~--~CH2 2-C~3<br />
CH3--~ CH 2 )IT"CH20H<br />
CH 3 CH 3 CH 3 CH 3<br />
I I I<br />
CH3-CK--~CH 2 3-CN--CH 2 3-CH--CN 2 3-C=CH2<br />
n=2-30 series<br />
CH3-~ CH 2 )~--CH3<br />
n=i-28 series<br />
CH2=CH--( CH 2 )~--CH 3<br />
CH3--( CH 2 )~4 COOH<br />
(226)<br />
12281<br />
(266)<br />
(58-450)<br />
(56-4341<br />
(256)<br />
14101<br />
fore <strong>and</strong> after Py-FIMS (error + 0.01 mg) to determine the<br />
pyrolysis residue <strong>and</strong> the produced volatile matter. <strong>The</strong><br />
heatable/coolable direct introduction system with elec-<br />
tronic temperature-programming, adjusted at the + 8 kV<br />
potential <strong>of</strong> the ion source <strong>and</strong> the field ionization emit-<br />
ter, was used. <strong>The</strong> slotted cathode plate serving as count-<br />
er electrode was on - 6 kV potential. Thus, at 2 mm dis-<br />
tance between the emitter tips <strong>and</strong> the cathode, in total a<br />
potential difference <strong>of</strong> 14 kV is applied resulting in an ex-<br />
tremely high electric field strength which is the essential<br />
basis for s<strong>of</strong>t ionization. All samples were heated in high<br />
vacuum (1.3 × 10 -4 Pa) from 323 K to 973 K at a heating<br />
rate <strong>of</strong> approximately 0.5Ks -1. About 60magnetic





![Legislativa - Označování potravin [režim kompatibility]](https://img.yumpu.com/15533670/1/190x135/legislativa-oznacovani-potravin-rezim-kompatibility.jpg?quality=85)







