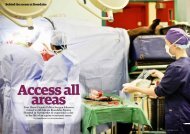
EQUINE CLINICAL PATHOLOGY - Rossdale & Partners
EQUINE CLINICAL PATHOLOGY - Rossdale & Partners
EQUINE CLINICAL PATHOLOGY - Rossdale & Partners
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
G u i d e t o e q u i n e c l i n i c a l p a t h o l o g y<br />
of tissue is then removed to include both<br />
adrenal glands and the adjacent aorta,<br />
for overnight fixation, prior to dissecting<br />
out the coeliaco-mesenteric sympathetic<br />
nerve ganglia, which lie between the<br />
adrenal glands and the aorta, but which<br />
are less easy to find in fresh condition.<br />
The bladder and internal genitalia are then<br />
removed and examined. The diaphragm<br />
is then examined and opened to reveal the<br />
thoracic viscera. The ribs are separated<br />
through their costochondral junctions,<br />
the intercostal muscles are cut and the<br />
ribs are broken back over the horse’s<br />
back. The ‘pluck’ including the trachea,<br />
heart and lungs are then removed for<br />
examination. The thymus is examined and<br />
removed in immature horses. The head<br />
is removed through the atlanto-occipital<br />
joint and ideally sectioned longitudinally<br />
with a band saw. The brain, meninges,<br />
guttural pouches, paranasal sinuses, teeth,<br />
pharynx and larynx are then examined<br />
thoroughly. The limb joints are opened<br />
for examination of the synovial fluid and<br />
surfaces. If indicated by the clinical signs,<br />
the neck and back vertebrae are dissected<br />
out and opened for examination of their<br />
articulations and the spinal cord. It is clear<br />
that a satisfactory examination of the head<br />
and spine of an adult horse is difficult to<br />
achieve under field conditions.<br />
Samples for bacterial or viral examination<br />
should be collected from abscesses, areas<br />
of inflammation, body cavities or seared<br />
viscera, using sterile swabs and placed<br />
without delay into Amies’ charcoal or<br />
specific viral transport media.<br />
Fluid samples from abscesses, cysts or<br />
body cavity fluids should be aspirated<br />
with a sterile syringe and then submitted<br />
in sequestrene (EDTA) for a nucleated cell<br />
count, and fixed with a suitable fixative<br />
(e.g. cytospin fixation fluid) for specific<br />
cytological processing. Another undiluted<br />
and unfixed sample should be submitted<br />
in a sterile container or on a sterile swab<br />
in transport medium or ideally into blood<br />
culture medium for concurrent bacterial<br />
culture.<br />
Samples for histopathological processing<br />
and examination should be carefully<br />
selected to provide thin and small, but<br />
representative tissue wedges, and totally<br />
immersed in 10% formol saline. Large<br />
thick samples fail to fix adequately and<br />
histopathological examinations are then<br />
unnecessarily complicated by autolytic<br />
changes, abused tissues may be ruined by<br />
artefactual damage and unrepresentative<br />
samples may not include the primary<br />
pathological changes.<br />
It is often wise to retain appropriate samples,<br />
e.g. gastric and intestinal content, urine and<br />
cubes of liver and kidney, carefully labelled,<br />
frozen, in case toxicological studies are<br />
required at a later date.<br />
49