Transoral CO2 laser microsurgery (TLM) - Vula - University of Cape ...
Transoral CO2 laser microsurgery (TLM) - Vula - University of Cape ...
Transoral CO2 laser microsurgery (TLM) - Vula - University of Cape ...
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
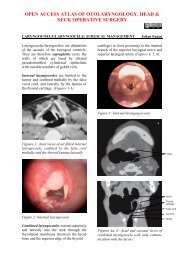
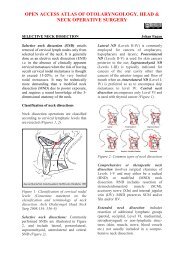
OPEN ACCESS ATLAS OF OTOLARYNGOLOGY, HEAD &NECK OPERATIVE SURGERYTRANSORAL LASER MICROSURGERY (<strong>TLM</strong>) OF CANCERS & OTHERPATHOLOGY OF THE UPPER AERODIGESTIVE TRACTJohan Fagan, Wolfgang SteinerCO 2 <strong>laser</strong> is used to resect or vaporisebenign and malignant lesions <strong>of</strong> the upperaerodigestive tract. This chapter focuses ontransoral CO 2 <strong>laser</strong> technique using amicromanipulator attached to an operatingmicroscope. The advantages <strong>of</strong> using CO 2<strong>laser</strong> with an operating microscope aremicrosurgical precision, excellent intraoperativedetail, and a dry surgical field; thefunctional outcomes in terms <strong>of</strong> swallowingand speech exceed that <strong>of</strong> traditionalexternal surgical approaches.Surgeons must familiarise themselves withthe <strong>laser</strong> machine, its settings and deliverysystems, and its tissue effects prior toattempting to use it clinically.1aArticulated armControl panelEmergency stopCO 2 <strong>laser</strong> machine (Figure 1)Surgical <strong>laser</strong>s convert radiation energyinto heat at the point <strong>of</strong> contact <strong>of</strong> thefocused radiation with the tissue. The <strong>laser</strong>beam is generated in a gas-containingdischarge tube. The light beam is collimated(coherent), is <strong>of</strong> a single wave-length(monochromatic), and can be reflected andhence manipulated by mirrors, and focusedby passing it through lenses. Because CO 2<strong>laser</strong> falls outside the visible light spectrumand is therefore invisible, the <strong>laser</strong>machine generates a red diode <strong>laser</strong> aimingbeam that is focused at the same workingdistance as the microscope to indicate tothe surgeon where the beam is directed.The <strong>laser</strong> beam is directed along a springloadedarticulated arm which connects to abeam applicator. The articulated arm hasmirrors at each articulation; therefore onehas to handle the articulated arm with greatcare so as not to disturb the alignment <strong>of</strong>the mirrors. The <strong>laser</strong> is fired when the footpedal is depressed.1bFigures 1a, b: CO 2 <strong>laser</strong>: Wet drapesprotect the patientSafety glassesFoot pedal
ightness <strong>of</strong> the aiming beam; power(Watts); and “Laser standby” or “Laserready” settings. Spot size is set with thefocus setting <strong>of</strong> the micromanipulator.Spot size0.2mm 0,4mm 0.8mm 1,4mm5 watts 16000 4000 1000 30010 watts 32000 8000 2000 60015 watts 48000 12000 3000 90020 watts 64000 16000 4000 1200Table 2: PD according to spot size andwatts 1Power (Watts)Figure 6: Example <strong>of</strong> <strong>laser</strong> control panelLaser Power Density (PD)Laser Power Density (PD) is a keysurgical parameter. It is affected by thedistance from the tissue, the diameter <strong>of</strong>the beam (<strong>laser</strong> spot size), and the number<strong>of</strong> Watts (Joules/sec); all these parameterscan be controlled by the surgeon.Key formulae to remember are:Power Density (PD) = Watts (W)/cm 2Watts (W) = Joules/secBy controlling PD the surgeon canmaximise the benefits <strong>of</strong> CO 2 <strong>laser</strong> surgery(Table 1).Power Effect on tissuesdensity0-500 Heating500-1500 Contracture, denaturing1500-5000 Ablation, partial vaporisation5000-20000 Incision, complete vaporisation20000-100000 Rapid deep incisionTable 1: Relationship between PD andtissue effects 1To achieve complete vaporisation, thesurgeon should aim for 4500 PD at thetissue interface. Table 2 illustrates howcritical the number <strong>of</strong> Watts and <strong>laser</strong> spotsize are to PD.This is selected by the surgeon every timethe <strong>laser</strong> machine is used, and can be preprogrammedfor different types <strong>of</strong> surgery.A <strong>laser</strong> machine with power settings <strong>of</strong> upto 40W is recommended for head and necksurgery.Laser spot sizeThe smaller the spot size, the less the range<strong>of</strong> depth <strong>of</strong> focus at which the <strong>laser</strong> cutseffectively and the more accurately the<strong>laser</strong> has to be focussed. Spot sizes <strong>of</strong> 0.5-0.8mm provide a comfortable compromisebetween depth <strong>of</strong> focus and cutting ability.To coagulate bleeding vessels, PD has tobe adjusted to a level at which tissue is nolonger vaporised, but is only heated andblood vessels are coagulated (Table 1);Tables 2 illustrates how increasing spotsize by defocusing the <strong>laser</strong> beam reducesthe PD.Total duty cycleThe total duty cycle is the total time that<strong>laser</strong> energy interacts with target tissues.This can be regulated in a number <strong>of</strong> ways(Figure 6):Continuous wave (CW): Continuousemittance <strong>of</strong> <strong>laser</strong> energy while the footpedal is depressedPulsed settings: Energy emitted asdiscrete pulses; the lengths, frequencies3
and time intervals between pulses canbe selected by the surgeono Single pulse: Single energy burstemitted every time the foot pedal isdepressedo Repeat pulse: Repeated energybursts emitted while the foot pedalis depressedo Super pulse (SP): Several thousandenergy bursts <strong>of</strong> very high peakpower <strong>laser</strong> pulses emitted persecond while the foot pedal isdepressed. The peak power <strong>of</strong> thebursts may be a few hundred watts,but the wattage shown on the <strong>laser</strong>machine reflects the average powertransferred over time. The burstsare spaced far enough apart forefficient tissue cooling to occurbetween pulses; this reducesthermal damage to surroundingtissue and minimises charring. Formore coagulation, switch from SPto CW.Laser/tissue interactionCO 2 <strong>laser</strong> is absorbed almost completelyby intracellular water and causesvaporisation <strong>of</strong> water and cells. Because99% <strong>of</strong> heat that is generated is lost in thevapour that is released, peripheral tissueinjury and necrosis are limited to
Because CO 2 <strong>laser</strong> is absorbed and“neutralised” by water, wet swabs andpatties are used and the cuff <strong>of</strong> theendotracheal tube may be filled withsaline. To reduce the risk <strong>of</strong> airway fire, itis critical that the anaesthetist keeps theFiO 2 at a minimum (+/- 30%) to avoid ablow-torch effect if the oxygen in theairways is ignited. It is advisable that anopen bowl <strong>of</strong> water/saline be kept at handto douse a <strong>laser</strong> fire.Anaesthesia for CO 2 <strong>laser</strong> surgeryThe principal anaesthetic challenges are touse endotracheal tubes that permit thesurgeon to work in the confined space <strong>of</strong>the larynx and pharynx, and to eliminatethe risk <strong>of</strong> <strong>laser</strong> fire (Table 3).Gases: Avoid nitrous oxide if possible andmaintain inspired oxygen FiO 2 as low as isclinically feasible; this requires continuousmonitoring <strong>of</strong> the patient’s oxygenation bypulse oximetry. The surgeon must notifythe anaesthetist before activating the <strong>laser</strong>and give the anaesthetist enough time toreduce the delivered oxygen concentrationto a minimum, to stop using nitrous oxide,and then to wait a few minutes for theoxygen concentration in the airways todrop to a safe concentration.Airway: The airway may be maintained ina number <strong>of</strong> ways:Endotracheal intubation (nasal or oral)Intermittent jet ventilation; <strong>laser</strong>ing isdone during apnoeic intervalsIntermittent extubation with <strong>laser</strong>ingdone during apnoeic intervals (2 ndauthor’s preference over jet ventilation)Open airwayo Spontaneous breathing <strong>of</strong> anaestheticgases administered throughthe suction port <strong>of</strong> the laryngoscopeo Intravenous anaesthesiaTracheostomy (rarely)It is important that the surgeon discusseswith the anaesthetist the optimal method <strong>of</strong>maintaining an airway for the specificpatient e.g. nasotracheal intubation may bebest for base <strong>of</strong> tongue cancer, but isproblematic for an endolaryngeal tumouras it fixes the position <strong>of</strong> the endotrachealtube.Endotracheal tubes: All tubes areflammable; no tube is therefore “safe”. Theproblem is not the type <strong>of</strong> tube, but perforatingthe tube when the O 2 concentrationin the tube is too high. The authors useregular PVC tubes, but take special care toprotect them with a strip <strong>of</strong> wet cloth cutfrom e.g. surgical drapes (Figure 7), orwith neuro patties.Figure 7: Tube protected by strip <strong>of</strong> wetclothThe drawbacks <strong>of</strong> “<strong>laser</strong> tubes” are thatthey have thicker walls and are expensive.PVC tubes with 5mm inner diameter aremore flexible, less expensive and pose noincreased risk for fire as long as the FiO 2 is
into the bronchi when a fluid-filled cuff isperforated.Surgical InstrumentationTo prevent reflection <strong>of</strong> the <strong>laser</strong> beam,instruments with beaded or mat surfacesare used (Figure 8). Adequate tumourexposure is critical to <strong>TLM</strong>; an integratedsuction channel to remove smoke isessential. It is therefore crucial that avariety <strong>of</strong> laryngoscopes and pharyngoscopesis available (Figure 8).Distending oropharyngoscope: Toaccess lesions in the tongue base,vallecula and lingual epiglottis; notethe side-flaps that keep the endotrachealtube and s<strong>of</strong>t tissues out <strong>of</strong> thesurgical field (Figures 8b, 10)abcgFigure 9: Distending laryngopharyngoscopedeeijhkflFigure 8: Laryngopharyngoscope (a), oropharyngoscope(b), laryngoscopes (c, d),light carrier (e), laryngoscope holder (f),graspers (g), insulated suckers (h), micr<strong>of</strong>orceps(i), coagulation forceps (j), diathermylead (k), and ligaclip applicators toleft and right (l) (Karl Storz)Distending laryngopharyngoscope: Toaccess lesions in the hypopharynx andsupraglottic larynx (Figures 8a, 9)Figure 10: Distending oropharyngoscopeLarge laryngoscope: To access endolaryngeal,upper tracheal and hypopharyngeallesions (Figures 8c, 11)Small laryngoscope: Laryngoscope issmaller and longer; used for difficultexposures such as anterior commissure,subglottis and upper trachea (Figures8d, 11)6
Figure 11: Larger & small laryngoscopesLight carrier (Figure 8e)Laryngoscope holder: (Figure 8f)Grasping forceps: Different sizes(Figure 8g)Suction tubes: insulated for monopolardiathermy (Figure 8h)Micr<strong>of</strong>orceps (for small vocal foldlesions): (Figure 8i)Coagulation forceps (Figure 8j)Diathermy cable: (Figure 8k)Liga clip applicators (left & right):(Figure 8l)Weerda diverticuloscope: For Zenker’sdiverticulum and for carcinoma <strong>of</strong> thehypopharynx extending to the upperoesophagus (Figure 12)Figure 13: Adjustable supporting plate forlaryngoscope holderOperating room setup (Figure 14)Figure 14: Typical operating room setupFigure 12: Weerda diverticuloscopeAdjustable supporting plate forlaryngoscope holder: This avoidsplacing pressure on the patient’s chest;the support can be moved away fromthe midline so as to angulate thelaryngoscope if necessary (Figure 13)A typical setup has the anaestheticmachine placed at the patient’s feet; thisrequires extensions for anaesthetic tubingand intravenous lines. A camera is attachedto the microscope so that the assistant andnurses can follow the procedure on amonitor. Two suction systems are required,one attached to the laryngoscope to extractsmoke from the surgical field and the otherattached to the hand-held suction tube.Monopolar diathermy should be availablefor <strong>laser</strong> procedures other than mid-cord T 1glottic carcinomas.7
Preparation <strong>of</strong> operative siteWound scrubs and paints should beaqueous or non-flammableEnsure that surgical prep solution doesnot puddle on or around the patientUse draping and gown material that isflame retardant or non-flammable, orwet it with waterSurround the scope with moistenedtowels, sponges or cotton to reduce thepossibility <strong>of</strong> fire or burns to thepatient’s facenot to disrupt the anterior commissure(Figure 16)Surgical exposureSurgical exposure is a critical element <strong>of</strong><strong>TLM</strong>. It may prove sometimes impossibleto proceed with <strong>TLM</strong> due to inadequateaccessPlace patient in supine position withneck extendedCheck that the head is resting on thebed and not suspended in mid-airCheck for loose or crowned teethInsert gum guardDo panendoscopy to exclude synchronousmalignanciesInsert laryngoscope / laryngopharyngoscope/ oropharyngoscope and exposethe tumourA number <strong>of</strong> different scopes may haveto be used to best visualise the tumourduring the course <strong>of</strong> an operationSuspend scope with the laryngoscopeholderIt may be necessary for the assistant topress on the larynx, or to place tapesattached to the theatre table across theneck to displace the larynx posteriorlyRarely an external approach is requiredfor access. This may be achieved bypassing the laryngoscope through asuprahyoid approach into the vallecula(Figure 15); or by doing a partiallaryng<strong>of</strong>issure, cutting only through theupper half <strong>of</strong> the thyroid cartilage so asFigure 15: Suprahyoid approach to thevalleculaFigure 16: View <strong>of</strong> glottic cancer via apartial laryng<strong>of</strong>issurePrinciples <strong>of</strong> CO 2 <strong>laser</strong> surgeryCO 2 <strong>laser</strong> may be used to incise, excise orvaporise (ablate) tissue. Novices shouldstart by operating on simpler cases e.g.smaller tumours <strong>of</strong> the aryepiglottic fold,supraglottis, or medial wall <strong>of</strong> piriformfossa. Important principles <strong>of</strong> CO 2 <strong>laser</strong>surgery include:1. Correct <strong>laser</strong> settings: Selecting spotsize, focus, power, and superpulse/pulsed/continuous mode, are importantin order to achieve the desired effects,and may have to be altered during the8
course <strong>of</strong> an operation for differenttissues, or to achieve coagulation,cutting or vaporisation effects etc.2. Tension: Constant traction must beapplied to the tissue to expose thedissection plane, maintain goodexposure and facilitate dissection3. Hand speed: The surgeon shouldmaintain a relatively slow, smoothhand speed4. Haemostasis:o Laser does not cut through blood;keep the field dry with suction /suction cautery / coagulation forceps/ liga clipso Use ligaclips, not suction diathermy,for large vessels to avoidpostoperative bleeding5. Charring: Charring is reduced bygenerating less heat by using the superpulsesetting. Wipe carbon <strong>of</strong>f thesurgical field with a wet cotton ball tomaximise the vaporisation effect ontarget tissue6. Minimise peripheral tissue injury:(Super)pulse as opposed to CW settingavoids excessive heating <strong>of</strong> tissue7. Avoid desiccation <strong>of</strong> tissues: Usenormal saline or water to wet tissues tobe <strong>laser</strong>ed as CO 2 <strong>laser</strong> interaction isimproved if tissue is well hydrated8. Test the alignment <strong>of</strong> the aiming beamwith that <strong>of</strong> the <strong>laser</strong> beam: This isimportant especially when using CO 2<strong>laser</strong> in otology as the margin for erroris much more critical; direct the <strong>laser</strong> ata wet, wooden tongue depressor tocheck the alignment9. Past-pointing: Avoid the possibility <strong>of</strong>past-pointing <strong>of</strong> the <strong>laser</strong> beam as thismay burn tissue or cause <strong>laser</strong> fires. Beaware <strong>of</strong> reflection <strong>of</strong> the <strong>laser</strong> beam<strong>of</strong>f instruments and scopes, and ensurethat the backstop e.g. neuro patty iscorrectly positioned to protect theendotracheal tube10. Resecting structures for improvedaccess: Resecting suprahyoid epiglottisimproves access to the pre-epiglotticspace and to tumours on the laryngealsurface <strong>of</strong> the epiglottis. Althoughresecting the false vocal cord improvesaccess to the laryngeal ventricle bothfor resection and follow-up, the benefit<strong>of</strong> improved access has to be weighedagainst possible functional deficits asthe preserved false vocal cord can beused to produce a good voice afterglottectomy. A small laryngoscopeintroduced from laterally will in mostcases adequately expose the ventricle;only with tumours spreading up tocartilage (T3) may it therefore benecessary to sacrifice the false vocalcord11. Debulking: This is a key <strong>TLM</strong> techniqueand allows the surgeon to createspace within which to move tissuesaround and to apply traction to tissue,or to resect a bulky tumour through anendoscope (Figure 17). The debulkedtissue is discarded, and only the deepertissue along the tumour margins aresent for histology to determine theadequacy <strong>of</strong> the resectionFigure 17: Example <strong>of</strong> tumour that hasto be debulked before oncologic resectioncan commence12. Hydrodissection: Water can be used asa barrier to penetration <strong>of</strong> the <strong>laser</strong>beam. Dissecting in the plane <strong>of</strong> Reinke’sspace or around nerves and vesselscan be facilitated by infiltrating salinesolution into Reinke’s space or intotissue planes around nerves and ves-9
sels. Haemostasis can be promoted byinjecting a solution containing epinephrine13. Interface between tumour and normaltissue (Figure 18): The surgeon distinguishestumour from normal tissuewhen transecting tumour by checkingthe pliability <strong>of</strong> the tissues (tumour isrigid), and by the colours <strong>of</strong> the tissues(tumour chars and is brown / blackwhen transected with <strong>laser</strong>)the patient. Specimens are pinned tosmall cut-outs <strong>of</strong> cork floor tiles thatare placed in formaldehyde so that thepathologist can orientate the specimen(Figure 20).Figure 19: Bread slicing the tumour toevaluate tumour depth and guide depth<strong>of</strong> resectionFigure 18: Note clear demarcationbetween dark brown transected tumourand pale-coloured normal tissue <strong>of</strong>paraglottic space14. Bread slicing: (Figure 19) In order toensure an adequate oncological deepmargin, but to minimise impairment <strong>of</strong>voice quality by not resecting too muchtissue, the surgeon needs to preciselydetermine the tumour depth and toresect along the deep aspect <strong>of</strong> thetumour under high magnification. Thisis achieved by serially sectioning(bread slicing) the tumour. This isapplicable to all tumours other thanvery superficial (T is or T 1 ) tumourswhich are simply peeled <strong>of</strong>f the vocalligament staying in Reinke’s space15. Orientating specimens: Take specialcare not to lose the orientation <strong>of</strong>specimens when removing them fromFigure 20: Specimens are pinned tocut-outs <strong>of</strong> cork floor tiles so thatpathologist can orientate the specimen16. Tumour topography: Include adetailed drawing on the pathology formand in the patient’s notes <strong>of</strong> the preciselocation <strong>of</strong> all the resected specimensWhat is an adequate resection margin?This is a controversial topic, and one thatmay cause surgeons a great deal <strong>of</strong> anxietyas to whether the patient should be returnedto the operating room for an additionalresection, whether simply to advise closesurveillance, or whether to recommendadjuvant irradiation. Factors that play arole in reaching such a decision include10
tumour location, size, function (voice andswallowing), patient fitness, extent <strong>of</strong> theinitial resection e.g. onto cartilage orcarotid, not knowing precisely where thepositive or close margin is located, and thereliability <strong>of</strong> regular follow-up. Frozen sectionmay be useful with larger resectionse.g. base <strong>of</strong> tongue and hypopharynx; yetpathologists are reticent to do frozen sectionson small resections e.g. T 1 glotticcancers, as it makes it difficult to commentdefinitively on adequacy <strong>of</strong> margins. Thesurgeon’s impression <strong>of</strong> the adequacy <strong>of</strong> aresection as seen through the microscope isalso important; he/she may elect to adopt awatchful waiting approach even whentumour is reported to be “present at themargin”, with the knowledge that cells aredenatured and killed adjacent to theincision during <strong>laser</strong> excision; such awatchful waiting approach applies especiallyto small mid-vocal fold carcinomasthat can be carefully monitored and reoperatedon timeously without adversely affectingprognosis.Glottic CarcinomaThe challenge for the <strong>laser</strong> surgeon is tostrike the correct compromise between anoncologically adequate resection and preservation<strong>of</strong> voice quality. The new <strong>laser</strong>surgeon should initially select smallertumours and progress to larger tumoursonly once he/she has gained a goodunderstanding <strong>of</strong> the endoscopic “insideout”surgical anatomy <strong>of</strong> the larynx, andhas become pr<strong>of</strong>icient with <strong>laser</strong> surgerytechnique. We do not advise voice restafter surgery except for superficial defects<strong>of</strong> the membranous cord.Classification <strong>of</strong> <strong>laser</strong> cordectomyThe European Laryngological Societyproposed a useful classification <strong>of</strong> endoscopiccordectomy in 2000 (Table 3,Figure 21) 2 .Cordectomy Type Resected tissueSubepithelial I EpitheliumSubligamental II Epithelium, Reinke's space,vocal ligamentTransmuscular III Through vocalis muscleTotalIVExtended Va Contralateral vocal fold,anterior commissureVb ArytenoidVc SubglottisVd VentricleTable 3: European Laryngological Societyclassification <strong>of</strong> endoscopic cordectomy 2Figure 21: Classification <strong>of</strong> cordectomy 2One can anticipate an excellent speakingvoice following Type I or subepithelialresection <strong>of</strong> T 1s and T 1 cancers <strong>of</strong> themembranous vocal fold (Figures 22, 23).Figure 22: Type I cordectomy; note intactvocal ligament11
Figure 23: Type I cordectomy: Pre- andpostoperativelyFigures 24 & 25 illustrate T1 glotticcancers that required Type II cordectomyand subsequent “new cord” formation dueto a scar band; such patients can beexpected to have a good, though not quitenormal quality voice.Figure 26: Type III cordectomyWith Type IV cordectomy the resectionextends onto thyroid cartilage with loss <strong>of</strong>volume <strong>of</strong> the hemilarynx and less predicttablevoice outcome (Figure 27).Figure 24: Type II cordectomyFigure 27: Type IV cordectomyThis applies to patients who undergo completeresection <strong>of</strong> the paraglottic space forT3 glottic carcinoma. Such patients mayhave to phonate using the false vocal cordsand may produce a good voice; hence thevalue <strong>of</strong> preserving the false vocal cords ifpossible.Figure 25: T1 glottic carcinoma requiredType II cordectomy; final resultFigure 26 illustrates transmuscular or TypeIII cordectomy for T 2 glottic carcinoma.This defect is subsequently filled by a scarband leading to a very adequate speakingvoice.Superficial lesions <strong>of</strong> membranous cordThe objective is to do a Type 1 resection,and to restore a normal speaking voice.Resection margins <strong>of</strong>
dissection with minimum lateral thermalinjury to the tissues. Surgery is done underhigh magnification. The initial incision ismade only through epithelium (Figure 28).thermal injury. Surgery is done under highmagnification. It is imperative to breadslicethe tumour in order to determine thedepth <strong>of</strong> the tumour and the deep resectionplane, and to remove it in sections (Figures18, 19, 30, 31).Figure 28: Initial incision for Type 1cordectomyThe cut edge <strong>of</strong> the epithelium is picked upwith micr<strong>of</strong>orceps and the tumour isdissected <strong>of</strong>f the vocal ligament, takingcare to remove and pin the tumour to thecork without losing spatial orientation(Figure 20).Figure 30: Example <strong>of</strong> sequence <strong>of</strong> incisionsfor a partial cordectomyDeeper lesions <strong>of</strong> membranous cordWith deeper tumours requiring Types II-IVresections the objective is to do an adequateresection (>1mm margin), and tomaintain an adequate speaking voice(Figure 29).Figure 31: Tumour has been transected todetermine depth <strong>of</strong> invasionFigure 29: Example <strong>of</strong> margins requiredSet the <strong>laser</strong> at its smallest spot size, power<strong>of</strong> 3-5 W and superpulse mode; this allowsa very precise dissection with minor lateralIt is generally easiest to 1 st remove theposterior segment, especially if access tothe anterior commissure if poor (Figure32).13
commissure with <strong>laser</strong> resection:Figure 32: Type 3 resection <strong>of</strong> posteriorhalf <strong>of</strong> tumourBleeding may be encountered especiallywhen dissecting adjacent to, or below, theanterior commissure and lateral to thevocal process <strong>of</strong> the arytenoid. Smallvessels may be coagulated by defocusingthe <strong>laser</strong> beam. More brisk bleeding iscontrolled using monopolar or bipolarcoagulation forceps. Avoid using suctiondiathermy on the vocal fold. Take care toremove and pin the tumour segments to thecork without losing spatial orientation, andto make a detailed drawing <strong>of</strong> theorientation <strong>of</strong> the segments in the patientrecords and on the pathology request form.It is also useful to paint the basal area(deep margin) blue, and to ask <strong>of</strong> thepathologist to determine whether thepainted area is free <strong>of</strong> tumour.Proximity to thyroid cartilage: Thismakes it difficult to obtain widemargins and raises the possibility <strong>of</strong>cartilage invasion (Figure 33). Theauthors do not routinely obtainimaging, but follow the tumour duringthe resection and may resect cartilagewith CO 2 <strong>laser</strong> if necessary. Once thevocal ligaments have been severedfrom the cartilage with the <strong>laser</strong>, thesurgeon can strip the tissues in a planedeep to perichondrium up to theinferior margin <strong>of</strong> the cartilage, andthen defocuses the <strong>laser</strong> and “sterilises”the cartilage <strong>of</strong> any residual cancercells (Figure 34).Figure 33: Extension through thyroidcartilageAnterior commissureThe authors do not consider anteriorcommissure cancer to be a contraindicationto <strong>laser</strong> resection. However some surgeonsconsider involvement <strong>of</strong> the anteriorcommissure to be a contraindication for<strong>laser</strong> resection and advocate vertical,supracricoid or even total laryngectomy.The following needs to be consideredwhen managing cancers <strong>of</strong> the anteriorFigure 34: Tissues stripped in subperichondrialplane and cartilage “sterilised”<strong>of</strong> cancer cells14
Subglottic tumour extension belowthyroid cartilage and through cricothyroidmembrane (Figures 35, 36):Webbing <strong>of</strong> anterior commissure causingpoor voice (Figure 37): Webbingoccurs when the anterior ends <strong>of</strong> bothvocal cords are denuded or resected(Figure 38). This can be avoided bydoing a two-stage resection, initiallyresecting only up to the midline, andcompleting the resection <strong>of</strong> the 2 ndvocal cord tumour about a month later(Figure 39).Figure 35: Subglottic extensionthrough cricothyroid membrane; endoscopicresection (red line)The surgeon follows the tumourendoscopically. Thyroid cartilage mayhave to be resected with <strong>laser</strong> to improveexposure and for resection <strong>of</strong>extralaryngeal tumour; resection mayextend to just deep to the skin <strong>of</strong> theneck if needed (Figure 36)Figure 37: Webbing <strong>of</strong> commissureFigure 38: Denuding both vocal cordsmakes webbing likelyFigure 36: Tumour escaping betweenthyroid and cricoid cartilagesIt may be difficult correct a web.Simply dividing the web with <strong>laser</strong>invariably leads to a new web. Anoption is to divide the web andendoscopic placement <strong>of</strong> a silastic keelfor 3 weeks (Figure 40) 3 , or to applyMitomycin C after excising <strong>of</strong> the scartissue with <strong>laser</strong>.15
Figure 39: Typical sequence <strong>of</strong> initial(blue) and 2 nd stage (red) resections <strong>of</strong>anterior commissure tumour to avoidwebbingFigure 41: Suture passed around vocalcord to lateralising the cordFigure 40: Silastic keel with holdingsutures 3An alternative which has beensuccessful in the 1 st author’s hands is todivide the anterior commissure and totemporarily lateralise the vocal cord(s).Two injection needles are passed fromthe outside <strong>of</strong> the neck through thyroidcartilage above and below the vocalcord. A suture is fed in and out <strong>of</strong> thelarynx through the needles, and thecord is lateralised by tying the suturestogether outside the neck for 3 weeks(Figure 41, 42).Supraglottic carcinomaSupraglottic carcinoma is resected using adistending laryngopharyngoscope (Figure9), graspers, and liga clip applicators toclip the superior laryngeal vessels that tra-Figure 42: Lateralised vocal cordverse the pharyngoepiglottic folds. Becausethe vocal cords are not beingoperated on, the surgeon sets the <strong>laser</strong> at ahigher power (>5W), slightly larger spotsize, and on superpulse mode so as to bothcut and coagulate. Surgery is done underhigh magnification, maintaining a distance<strong>of</strong> >5mm from the edge <strong>of</strong> the tumour.Provided a neck dissection is not donesimultaneously, antibiotics are notgenerally required.Cancer <strong>of</strong> suprahyoid epiglottisThe suprahyoid epiglottis can be resectedwith only transient and relatively minordifficulty with swallowing (aspiration) thatsettles once postoperative pain resolves.16
Cancer <strong>of</strong> infrahyoid epiglottis and falsevocal cordsPostoperative aspiration is minor whencompared to open supraglottic laryngectomy;the nasogastric tube is usually removedwithin a few days. The resection isadapted to the tumour and may e.g. involveonly removing one half <strong>of</strong> the epiglottisInsert a distending laryngopharyngoscopewith the anterior blade in thevallecula, and open it widelyDetermine the extent <strong>of</strong> the tumour andplan the resectionRemove the suprahyoid epiglottis forexposure and to remove the suprahyoidcomponent <strong>of</strong> the tumouro Make a curved incision in the vallecula(Figures 43, 44)o Bisect the suprahyoid epiglottis in asagittal plane, cutting throughtumour if need be (require higherpower setting to cut through epiglottis(Figure 43)o Cut transversely across theepiglottis at the level <strong>of</strong> the hyoid(Figure 44)o Remove the left and/or right segments<strong>of</strong> the suprahyoid epiglottisFigure 43: Initial incision in thevallecula and though the epiglottisReadjust the position <strong>of</strong> the distendinglaryngopharyngoscope to improveaccess to the infrahyoid component <strong>of</strong>the tumourFigure 44: Suprahyoid (blue) andinfrahyoid (red) incisions for <strong>laser</strong>supra-glottic laryngectomy; preepiglotticfat (yellow)Incise the pharyngoepiglottic folds,taking care to identify the branches <strong>of</strong>the superior laryngeal vessels. Althoughsmall vessels can be coagulatedwith the angled grasping coagulationforceps, resist the temptation to coagulatethe superior laryngeal artery withelectrocautery; rather apply 2 ligaclipsto the artery before dividing it with<strong>laser</strong>Preepiglottic dissection (Figure 44)o Cut transversely into the avascularpreepiglottic fat, preserving a layer<strong>of</strong> fat on the anterior aspect <strong>of</strong> thespecimen as a resection margino Palpate with the sucker to determinethe position <strong>of</strong> the hyoid boneand the superior edge <strong>of</strong> the thyroidcartilageo Cut down onto and expose thethyroid cartilageBisect the infrahyoid epiglottis in asagittal plane, cutting through tumourif need be (require higher power settingto cut through epiglottis)17
Follow the tumour e.g. through thethyrohyoid membrane; thyroid cartilagemay also be resectedRemove tumour in as many segmentsas is required, taking care to maintaintheir orientation for the pathologistFrozen section may be used to directthe extent <strong>of</strong> the resectionSecure haemostasis with monopolarsuction cautery or coagulation forceps,preferably bipolarFigures 45a-d & 46a-c illustrate supraglotticcancers, their excision and recovery.Figure 45c: Completed resectionFigure 45a: (L) supragottic cancerFigure 45d: Following healingaFigure 45b: Resecting the 1 st segment;note endotracheal tube has been displacedanteriorly to improve visibility for theposterior resectionFigures 46 a: Supraglottic cancer extendingonto right aryepiglottic andpharyngoepiglottic folds18
Figures 46 b: Completed resectionbWhether lateral wall tumour extendsbehind thyroid lamina (Figures 47, 48)o Proximity <strong>of</strong> carotid sheatho Need to delay neck dissections by a2 weeks to avoid thru’-and-thru’fistula into the neckThe distal extent <strong>of</strong> tumour as oneshould attempt to avoid a circumferentialresection at the narrow cricopharyngeusregion as this may cause astricturecFigures 46 c: Following healingHypopharyngeal carcinomaCancers <strong>of</strong> the piriform fossa may extendmedially into paraglottic space, cricoid andcricoarytenoid joint; anteriorly into preepiglotticspace; and laterally to invadethyroid cartilage, and behind thyroidlamina, to the s<strong>of</strong>t tissues <strong>of</strong> the neck andthe carotid sheath (Figure 47).Figure 47: Extension <strong>of</strong> piriform fossacancer: red arrows indicate higher riskresection due to proximity to carotidAt diagnostic endoscopy assessDepth and mobility <strong>of</strong> tumouro Tumours clearly limited to mucosaare easily resectedo Deeply invasive tumours <strong>of</strong> thelateral wall need additional CT +/MRI imaging as deeply invasivetumours infiltrating s<strong>of</strong>t tissuearound the carotid artery are notamenable to <strong>laser</strong> excisionFigure 48: Posterior edges <strong>of</strong> thyroidlaminae19
Resection techniqueSurgery is done under high magnificationthrough laryngoscopes and a distendinglaryngopharyngoscope (Figure 9) usinggraspers, and liga clip applicators to clipthe superior laryngeal vessels that traversethe pharyngoepiglottic folds. Maintain adistance <strong>of</strong> 5-10mm from the mucosal edge<strong>of</strong> the tumour. Because the vocal folds arenot being operated on, the surgeon sets the<strong>laser</strong> at a higher power (>5W), slightlylarger spot size, and on CW mode so as toboth cut and coagulate tissue. Reduce thepower when dissecting posterior to thethyroid lamina in the region <strong>of</strong> the carotidsheath. Both authors have on occasionexposed the carotid artery during resectionfor hypopharyngeal cancers without anyadverse effects; the vessel was either leftuncovered or sealed with fibrin tissue glue.The tumour is excised in segments, takingcare to orientate the specimens relevant forhistology <strong>of</strong> the deep margins for the pathologist.With major resections insert anasogastric feeding tube under directvision to avoid the tube transgressing thetumour bed and entering the s<strong>of</strong>t tissues <strong>of</strong>the neck, or arrange for a PEG. Provided aneck dissection is not done simultaneously,antibiotics are not required.Figure 49: Postcricoid cancer with extensionto piriform fossa (a) and followingresection and healing (b)babFigures 49 and 50 show examples <strong>of</strong>piriform fossa and postcricoid cancers bothbefore resection and after healing haveoccurred.aFigure 50: Piriform fossa cancer withextension to postcricoid area beforeresection (a) and following healing (b)20
Base <strong>of</strong> tongue (BOT) (Figures 51, 52)abFunctional outcomes are superior toexternal surgical resections. Depending onanatomical factors, accessing BOTtumours for <strong>laser</strong> excision may be veryeasy or impossible (other than in the hands<strong>of</strong> the expert using small scopes.Figure 51: Cancer <strong>of</strong> BOT (a) and followingresection and healing (b)Key issues relating to BOT cancerresection:Obtain a CT, or preferably an MRIscan, both in axial and sagittal planesto assesso Tumour deptho Anterior deep extension <strong>of</strong> tumouro Involvement <strong>of</strong> preepiglottic fatInsert a nasotracheal tube to improveaccess and protect it with a wet cloth ora neuro pattyApply anterior traction to the tonguewith a suture passed through the tongueaOral and oropharyngeal lesions may beexcised with CO 2 <strong>laser</strong> using the microbFigure 52: Preoperative (a) andpostoperative scan showing absentepiglottis and reduced volume <strong>of</strong> BOT (b)Use a distending oropharyngoscope(Figures 10a, b) +/ large laryngoscope+/ tonsil gag for accessWith long procedures, relieve pressure<strong>of</strong> the scope from time-to-time torestore blood circulation to the tongueDo not sacrifice both lingual arteries asthe tongue may infarctDo not sacrifice both hypoglossal nervesas the patient will then be an oralcripple (dysphagia and dysarthria)Resect the tumour in multiple segmentsFrozen section is used to guide the surgeryas margins may not be as clearlyvisible as with laryngeal cancerOrientate the resected segments accuratelyfor the pathologist to commenton marginsNo flaps are required, as the defectheals spontaneouslyTracheostomy is generally not requiredWith big resections that include resectingthe epiglottis, a nasogastric tube isinserted for feedingWhen expecting persistent deglutitionproblems e.g. with extended resectionsfor large tumours requiring adjuvantradiotherapy, a PEG should be consideredearlyOral cavity and oropharynx21
scope. All lesions should be excised forhistological evaluation i.e. not vaporised.Figures 53a & b illustrate the excellentfunctional outcome after leaving a tumourbed to heal following <strong>laser</strong> excision.Figures 54a & b illustrate resection <strong>of</strong>cancer <strong>of</strong> the s<strong>of</strong>t palate.baFigures 54a, b: Cancer <strong>of</strong> s<strong>of</strong>t palate, tonsilsand posterior pharyngeal wall before(a) and after resection and healing (b)bCO 2 LASER FOR BENIGN DISEASELaryngocoeles and saccular cystsInternal laryngocoeles and saccular cystsdo very well with wide marsupialisationwith CO 2 <strong>laser</strong> and removing the contents<strong>of</strong> the cyst with a sucker (Figure 55, 56).Figure 53: Cancer tongue before (a) andafter resection and healing (b)aFigure 55: Saccular cyst suited to CO 2<strong>laser</strong> marsupialisation22
Papillomata at the anterior commissurepresent a challenge in terms <strong>of</strong> avoidingwebbing and a poor voice. One may electto stage the treatment at the anteriorcommissure by ablating only one side at atime to avoid webbing. Tracheal papillomatacan be ablated using the microscopeby advancing a smaller and longer laryngoscopebetween the vocal cords; or by usinga <strong>laser</strong> bronchoscope (Figure 3).Intubation granulomaFigure 56: Inside surface <strong>of</strong> large internallaryngopyocoele following marsupialisationwith <strong>laser</strong>Intubation granulomas originate on thetraumatised vocal process <strong>of</strong> the arytenoid(Figure 58).Viral papillomatosis (Figure 57)The objectives <strong>of</strong> surgery for viralpapillomatosis are not to eradicate allvirally infected tissue, but to maintainairway and voice. The most populartechniques are CO 2 <strong>laser</strong> and microdebriders.Ablation <strong>of</strong> papillomata is donewith CO 2 <strong>laser</strong> on a low power setting andby slightly defocusing the beam to create alarger spot size. Surgery should beconservative to preserve voice.Figure 58: Intubation granulomaFigure 57: Viral papillomataFigure 59: Excised granuloma23
Only if they do not resorb after a fewmonths and remain symptomatic are theyremoved, taking care not to expose thecartilage so as to reduce the likelihood <strong>of</strong>recurrence (Figure 59).Paralysed vocal cordsWith surgery done to relieve airwayobstruction due to bilateral vocal foldparalysis, the patient has to be forewarnedabout the trade-<strong>of</strong>f <strong>of</strong> airway vs. voicequality, and that a 2 nd operation may berequired should the airway still not beadequate. While some surgeons prefer initiallyto do unilateral posterior cordectomy,the 2 nd author routinely does bilateralposterior cordectomy as in his experiencethis yields a good outcome both in terms <strong>of</strong>airway and voice.The surgery is done around a small (5mm)endotracheal tube with the laryngoscopeplaced behind the tube so as to expose theposterior larynx. CO 2 <strong>laser</strong> is used to exciseonly the vocal process <strong>of</strong> the arytenoid(posterior 1/3 <strong>of</strong> vocal fold) and a variableamount <strong>of</strong> tissue lateral to the vocalprocess (Figure 60). It is important that theanterior 2/3 (vibrating part) <strong>of</strong> the vocalfold be preserved to optimise voice quality.The final result is only apparent monthsafter the surgery once fibrosis and healinghave matured. The voice quality is usuallyvery adequate as there is improved airflowthrough the larynx due to the larger airway,and because surgery is limited to theposterior glottis (Figure 61).Figure 61: Final result <strong>of</strong> unilateralposterior cordectomySubglottic and tracheal stenosisCO 2 <strong>laser</strong> is best suited to thin webs. Thesurgery is done by passing the smaller,longer laryngoscope past the vocal folds.Anaesthesia is done with an open airway,either having the patient breathing spontaneouslywith intravenous anaesthesia, orwith intermittent jet ventilation; the 2 ndauthor prefers using intermittent extubationwith the surgery done during apnoeicintervals. The stricture is incised in a radialfashion, preserving mucosal bridgesbetween the cuts (Figures 62).Figure 60: Bilateral posterior cordectomyFigure 62: Tracheal stenosis; preservedmucosal bridges24
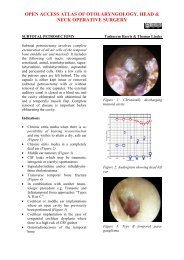
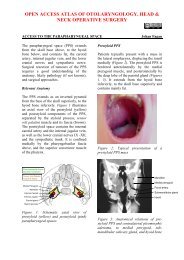
Pharyngeal pouch (Zenker’s diverticulum)Endoscopic diverticulotomy involves dividingthe “party wall” between the oesophagusand the diverticulum, as well as thecricopharyngeus muscle which is locatedwithin the superior part <strong>of</strong> the “party wall”(Figure 63). It may be performed with astapler (Figure 64) or with CO 2 <strong>laser</strong>. The2 nd author (W.S) has preferred CO 2 <strong>laser</strong>endoscopic diverticulotomy for the past 30years. Both techniques have proven to beeffective and safe.Figure 63: Party wall that contains cricopharyngeusmuscle separates oesophagus(anterior) from pouch (posterior)Figure 65: Large and small diverticulaCO 2 <strong>laser</strong> is particularly useful for pouches<strong>of</strong>
Figure 67: Perforated pouchSurgical steps for <strong>laser</strong> diverticulotomyDetermine the anatomy <strong>of</strong> the pouchand the location <strong>of</strong> the opening <strong>of</strong> theoesophagus with rigid oesophagoscopyPass the closed end <strong>of</strong> the Weerdascope into the pouch taking care not toperforate the thin wall <strong>of</strong> the pouchOpen the blades, and retract the scopeuntil the oesophageal opening appearsanteriorly (A trick to make it easier tolocate the oesophageal opening is t<strong>of</strong>irst place a suction catheter in theoesophageal opening)Pass the anterior blade <strong>of</strong> the Weerdascope into the oesophagus and keep theposterior blade in the pouchDistract the blades further to bring theparty wall into view, and suspend thescopeClear the pouch <strong>of</strong> food debrisInsert a wet strip <strong>of</strong> cloth into theoesophagus to protect it from injurydue to past pointing <strong>of</strong> the <strong>laser</strong>Set the <strong>laser</strong> at 5W, CW mode andsmall spot sizeTransect mucosa and cricopharyngeusmuscle <strong>of</strong> the party wall (Figure 68)Stop the dissection 5mm from thefundus <strong>of</strong> the pouchBroad spectrum antibiotics are givenperioperativelyFigure 68: Divided party wallIntroduce free fluids on Day 1; progressto a s<strong>of</strong>t and then solid diet overthe course <strong>of</strong> a week (Some surgeonselect to insert a nasogastric feedingtube for 2 days)Complications <strong>of</strong> <strong>laser</strong> surgeryPatients have surprisingly little painfollowing <strong>laser</strong> resections. They commonlymention having to clear their throats for afew weeks due to discharge from thehearing surgical bed.Early complicationsDental: Use a gum guard to protect theteeth, and check for crowns or implantsand explain the risks to the patientbefore surgeryOropharyngeal trauma: It is notuncommon to cause minor tears <strong>of</strong> thetonsillolingual mucosa when havingdifficulty with endoscopic accessLingual nerve: Prolonged upwardtraction on the base <strong>of</strong> the tongue cancause transient sensory change <strong>of</strong> theoral tongue due to traction injury to thelingual nerveBleeding: It is important to apply ligaclips, and not to cauterise, majorvessels. Though infrequent, this can be26
a catastrophic event, especially when itoriginates from the lingual, superiorlaryngeal or carotid arteriesSurgical emphysema: This occursmainly in the setting <strong>of</strong> surgery in thesubglottic region e.g. resection throughthe cricothyroid membrane, and istreated conservatively; it occurs withsmall defects, as air is then trapped inthe s<strong>of</strong>t tissues. To avoid emphysemaone can press on the larynx duringextubation and subsequently apply abandage around the neckAirway obstruction: This is anuncommon event; hence prophylactictracheostomy is only rarely indicatedAspiration: This does not occur withcordectomies, but may complicatesupraglottic largyngectomy and BOTresections. It is rarely a persistentproblem, and occurs less frequentlythan with open resections. Initial managementmay include good paincontrol, thickened foods (rather thanliquids); and temporary nasogastrictube or PEG feedingLaser burns: This occurs when thetissues e.g. facial skin are not properlyprotected with wet drapesAirway fire: This is an extremely rareevent and is entirely preventableLate complicationsGranuloma (Figure 69): This occursespecially where cartilage has beenexposed e.g. at the anterior commissureand the vocal process <strong>of</strong> the arytenoid.It generally resolves with time, butmay require biopsy if it cannot bedistinguished from tumour recurrenceChondronecrosis: This rarely occurswhen cartilage has been exposed or excised,especially after salvage surgeryin a post chemoradiation setting. Thepatient is treated with antibiotics andmicrolaryngoscopy to remove mucusand sequestra, and by sterilizing theremaining cartilage with the <strong>laser</strong> toimprove healing and to excluderesidual tumour (Figure 70)Figure 69: Granuloma on arytenoidFigure 70: Chondronecrotic sequestraStricture: This occurs very rarely withtumour surgery. Figures 71a-c illustratea patient who developed a completestricture in the region <strong>of</strong> the cricopharyngeusfollowing circumferentialexcision <strong>of</strong> a tumour <strong>of</strong> the hypopharynx;the stricture was bypassedwith a jejunal free flap27
abSuggested ReadingEndoscopic Laser Surgery <strong>of</strong> the UpperAerodigestive Tract: With Special Emphasison Cancer Surgery. W Steiner, PAmbrosch (2000) ISBN-10: 08657-7996, ISBN-13: 978-0865779969<strong>Transoral</strong> Laser Microsurgery for Cancer<strong>of</strong> the Upper Aerodigestive Tract(incl. DVD): Steiner W. 2013 (Distributedat no charge by Karl Storz, Tuttlingen)American Society for Laser Medicineand Surgeryhttp://www.aslms.org/public/standardpsgs.shtmlApfelbaum JL et al. Practice advisoryfor the prevention and management <strong>of</strong>operating room fires: an updated reportby the American Society <strong>of</strong> AnesthesiologistsTask Force on OperatingRoom Fires. Anesthesiology. 2013Feb;118(2):271-90cReferences1. Peter H. Eeg. CO 2 Lasers: The FourKeys to Success. Veterinary PracticeNews Posted: May 23, 2011http://www.veterinarypracticenews.com/vet-dept/small-animal-dept/c02-<strong>laser</strong>s-the-four-keys-to-success.aspx2. Remacle M et al. Endoscopic cordectomy.A proposal for a classification bythe Working Committee, EuropeanLaryngological Society. Eur ArchOtorhinolaryngol. 2000;257(4):227-313. Lichtenberger G, Toohill RJ. New keelfixing technique for endoscopic repair<strong>of</strong> anterior commissure webs. Laryngoscope1994:104 (6):771–4Figures 71a-c: Stricture <strong>of</strong> hypopharynx(a); inset <strong>of</strong> jejunal free flap(b); and postoperative result (c)28
Author and EditorJohan Fagan MBChB, FCORL, MMedPr<strong>of</strong>essor and ChairmanDivision <strong>of</strong> Otolaryngology<strong>University</strong> <strong>of</strong> <strong>Cape</strong> Town<strong>Cape</strong> Town, South Africajohannes.fagan@uct.ac.zaAuthorWolfgang Steiner MD, Hon. FRCS (Engl)Pr<strong>of</strong>essor em. & Past ChairmanDept <strong>of</strong> ENT, Head & Neck Surgery<strong>University</strong> <strong>of</strong> GoettingenGöttingen, Germanywolfgang.p.steiner@googlemail.comTHE OPEN ACCESS ATLAS OFOTOLARYNGOLOGY, HEAD &NECK OPERATIVE SURGERYwww.entdev.uct.ac.zaThe Open Access Atlas <strong>of</strong> Otolaryngology, Head &Neck Operative Surgery by Johan Fagan (Editor)johannes.fagan@uct.ac.za is licensed under a CreativeCommons Attribution - Non-Commercial 3.0 UnportedLicense29