Acids & Bases - Chemistry
Acids & Bases - Chemistry
Acids & Bases - Chemistry
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Acids</strong> & <strong>Bases</strong> 6SubstancepH10 M HCl −1.01 M HCl 0.0Stomach Acid (HCl) 1.4Lemon Juice 2.1Orange Juice 2.8Wine 3.5Black Coffee 5.0Urine 6.0Pure Water 7.0Blood 7.4Baking Soda Solution 8.5Ammonia Solution 11.91 M NaOH 14.010 M NaOH 15.0
<strong>Acids</strong> & <strong>Bases</strong> 7We can also define:pOH = −log [OH − ]Although most chemists mainly use pH, pOH canbe useful in base equilibrium numerical problemsthat we will run into later.Another definition we use is:pK w = −log K w = 14So, for a given water solution:or:pOH + pH = pK w = 14pOH = 14 − pHpH = 14 − pOH
<strong>Acids</strong> & <strong>Bases</strong> 8Example: The pH of wine is 3.5. What is the[H + ]? What is the [OH − ]? What is the pOH?pH = −log[H + ] = 3.5[H + ] = antilog(−3.5) = 10 −3.5 = 3.16 × 10 −4 M−14− Kw1×10−11[ OH ] = = = 3.1×10−4[ H+] 3.16×10pOH = −log[OH − ] = −log(3.1 × 10 −11 ) = 10.5---- or ----pOH = pKw − pH = 14 − 3.5 = 10.5Problem: The pH in your stomach is around 1.What is the [H + ]? What is the [OH − ]? What is thepOH?
Dissociation Equilibrium Constants<strong>Acids</strong> & <strong>Bases</strong> 9An acid is a compound that will ionize in solution(usually water) to form a H + (aq) and a counteranion.This can be writen in a general fashion as:HA(aq) H + (aq) + A − (aq)The equilibrium expression for this reaction is:+ −Keq=[ H ][A ][ HA]The equilibrium constant, K eq , is often given aspecial name for acids: K a or the acid dissociationconstant. The larger the K a value the more H + isbeing produced (the lower the corresponding pH),therefore, the stronger the acid!!!!Just as with pH we can also define pK a as:pK a = −log K aThe smaller (and more negative) the pK a thestronger the acid!!!! This can be confusing, but isvery important: pK a ’s are commonly used inbiology & chemistry!
Acid Dissociation Constants and pK a Values<strong>Acids</strong> & <strong>Bases</strong> 10Acid HA A - K a pK ahydroiodic HI I − ∼10 10 ∼ −10hydrobromic HBr Br − ∼10 10 ∼ −10perchoric HClO 4 ClO 4−∼10 8 ∼ −8hydrochoric HCl Cl − ∼10 8 ∼ −8sulfuric H 2 SO 4 HSO 4−∼10 8 ∼ −8nitric HNO 3 NO 3−∼10 8 ∼ −8trichloroacetic Cl 3 COOH Cl 3 COO − 2 × 10 −2 0.7oxalic HOOCCOOH HOOCCOO − 5.9 × 10 −2 1.2sulfurous H 2 SO 3 SO 32−1.5 × 10 −2 1.8sulfuric (2nd H + ) HSO 4- SO 42−1.2 × 10 −2 1.9phosphoric H 3 PO 4 H 2 PO 4−7.5 × 10 −3 2.1nitrous HNO 2 NO 2− 4.6 × 10 −4 3.3hydrofluoric HF F − 3.5 × 10 −4 3.5formic HCOOH HCOO − 1.8 × 10 −4 3.8benzoic C 6 H 5 COOH C 6 H 5 COO − 6.5 × 10 −5 4.2oxalic (2nd H + ) HOOCCH 2 COO − − OOCCH 2 COO − 6.4 × 10 −5 4.2acetic CH 3 COOH CH 3 COO − 1.7 × 10 −5 4.7carbonic H 2 CO 3 HCO 3− 4.3 × 10 −7 6.4hydrogen sulfide H 2 S HS - 9.1 × 10 −8 7.1phosphoric (2nd) H 2 PO 4− HPO 42−6.2 × 10 −8 7.2ammonium ion NH 4+ NH 3 5.6 × 10 −10 9.2hydrocyanic HCN CN - 4.9 × 10 −10 9.3
Strong <strong>Acids</strong>These are acids that are essentially completelydissociated in solution (usually water).HCl(aq) H + (aq) + Cl − (aq)[ H+][ Cl−]8Ka= ≈[ HCl]10<strong>Acids</strong> & <strong>Bases</strong> 11In general, K a > 1 for a strong acid (although thereis no firm dividing line!)The common strong acids that you are expected toknow are:HCl, HBr, HI (the hydrohalic acids)H 2 SO 4 (sulfuric acid) HNO 3 (nitric acid)HClO 4 (perchloric acid)HCl, H 2SO 4, and HNO 3are often referred to as mineral acids.A strong acid completely dissociates the first H + , sothe H + concentration is the same as the given acidconcentration. ∴ the pH of a strong acid is just−log of the acid concentration (= −log[H + ]).
<strong>Acids</strong> & <strong>Bases</strong> 12Problem: What are the pH's of the followingsolutions?a) 0.001 M HClb) 0.1 M HNO 3c) 1 × 10 −5 M H 2 SO 4d) 10 M HBre) 1 M HIf) 0.1 M HFg) 0.01 M HClh) 1 × 10 −4 M HNO 3i) 1 M acetic acidj) 1 × 10 −14 M HCl
<strong>Acids</strong> & <strong>Bases</strong> 13Explanation for j) on the last page:The actual proton concentration for any aciddissolved in water is more precisely defined as:[H + ]total = [H + ]acid + [H + ]waterNormally, [H + ]water is 1 × 10 −7 M and is much lessthan [H + ]acid, so that we usually ignore it.BUT, in this example, [H + ]acid turns out to beonly 1 × 10 −14 M, which is much, much less thanthe [H + ]water.So in this example we can actually ignore the tinyamount of H + contributed from the strong acid andonly consider the H + naturally present in water:This will work for acid concentrations of 1 × 10 −8 M andlower. It gets complicated mathematically right around1 × 10 −7 M (not dealt with in this course).
<strong>Acids</strong> & <strong>Bases</strong> 14Lewis Dot Structures: Where’s the Proton?When we write the formula for HNO 3 , it does NOTmean that the proton (H + ) is attached to thenitrogen atom. The proton always binds to, or isassociated with a lone pair of electrons, usually onone of the outer negatively charged atoms of apolyatomic anion. Consider the Lewis Dotstructures for NO 3 − , SO 4 2− , and ClO 4 − :when N has 4 bonds,it is formally cationicScanhavemore than 8 ve-Cl can havemore than 8 ve-OO2−OONOOSOOOClOOnitrate anionsulfate dianionperchlorate anionResonance, of course, will spread out the negativecharges and bonding over all the O atoms. H + ineach of these cases binds to the oxygen atom(s) thatis (are) negatively charged:OOOONH O S O H O Cl O HOO HOnitric acidsulfuric acidperchloric acid
<strong>Acids</strong> & <strong>Bases</strong> 15Problem: Draw the Lewis Dot structure that bestminimizes the formal charges for the followingacids (some strong, some weak). Cleary showwhere the H + is coordinated.a) HBF 4 (fluoroboric acid, strong)b) H 2 CO 3 (carbonic acid, weak)c) H 3 PO 4 (phosphoric acid, weak)d) CF 3 SO 3 H (triflic acid, strong)e) HCO 2 H (formic acid, weak)
Strong & Weak vs. pH<strong>Acids</strong> & <strong>Bases</strong> 16A common mistake is to confuse pH with strong orweak acids/bases. For example, if I tell you asolution has a pH of 5.0, most of you wouldincorrectly assume that it is a weak acid. Maybe,maybe not.A pH of 5.0 tells you that it is a weakly acidicsolution. This solution might have been madefrom a medium to considerable amount of a weakacid, or a very small amount of a strong acid.Without knowing the concentration (or amountand nature) of the acid used (not just the resultingH + concentration or pH), you can’t tell whether it iscomposed of a strong or weak acid.It is true that solutions with very low pH’s (forexample, −1.0) can pretty much only be composedof strong acids.So you need to be very careful with your languagein dealing with strong & weak acids and solutionsthat are strongly or weakly acidic.
Polyprotic <strong>Acids</strong>Sulfuric Acid:<strong>Acids</strong> & <strong>Bases</strong> 17H 2 SO 4 (aq) H + (aq) + HSO − 4 (aq) K a1 ≅ 10 8HSO − 4 (aq) H + (aq) + SO 2− 4 (aq) K a2 ≅ 10 -2Phosphoric Acid:H 3 PO 4 (aq) H + (aq) + H 2 PO 4 − (aq) K a1 ≅ 10 -3H 2 PO 4 − (aq) H + (aq) + HPO 4 2− (aq) K a2 ≅ 10 -8HPO 2− 4 (aq) H + (aq) + PO 3− 4 (aq) K a3 ≅ 10 -13Carbonic Acid:H 2 CO 3 (aq) H + (aq) + HCO − 3 (aq) K a1 ≅ 10 -7HCO 3 − (aq) H + (aq) + CO 3 2− (aq) K a2 ≅ 10 -11
<strong>Acids</strong> & <strong>Bases</strong> 18Note that for polyprotic acids only the firstdissociation (the first H + ) is likely to occur. Thesecond (or third) dissociation process is far lesslikely, so the contribution of these subsequentdissociations to the overall [H + ] total .So, generally we only have to worry about thefirst proton and the first dissociation constantunless one is doing a titration or other acidbasereaction!!
<strong>Acids</strong> & <strong>Bases</strong> 19Strong <strong>Bases</strong>These are usually (and most commonly) alkalimetal hydroxides that dissociate completely insolution:NaOH(aq)Kb+[ Na][ OH]= ≈[ NaOH]Na + (aq) + OH − (aq)−10 8The common strong bases that you are expected toknow are:LiOH, NaOH, KOH, RbOH, & CsOHThe alkaline earth hydroxides Ca(OH) 2 , Sr(OH) 2 ,Ba(OH) 2 are medium strong bases.Be(OH) 2 & Mg(OH) 2 are considered to be weakbases since they only partially dissociate in water.The smaller ionic radius of Be 2+ and Mg 2+ cationspolarize coordinated H 2 O enough to promotehydrolysis (that is, loss of H + from H 2 O).
<strong>Acids</strong> & <strong>Bases</strong> 20It is very important to remember thatthere are almost always two steps inconverting from [OH − ] to pH:1) convert [OH − ] to [H+]:K[ H+ w] =[ OH − =]2) then convert [H + ] to pH.---- or ----1) convert [OH − ] to pOH2) then convert pOH to pH:−1×10[ OH− ]pH = 14 − pOH14
<strong>Acids</strong> & <strong>Bases</strong> 21Problem: What are the pH's of the followingsolutions? What are the pOH's?a) 0.001 M NaOHb) 0.1 M CsOHc) 1 × 10 −5 M KOHd) 10 M RbOHe) 1 M NaOHf) 0.1 M Be(OH) 2g) 0.01 M LiOHh) 1 × 10 −4 M NaOHi) 1 M ammoniaj) 1 × 10 −14 M KOH
<strong>Acids</strong> & <strong>Bases</strong> 22Explaination for j) on the last page:The actual hydroxide concentration for any basedissolved in water is more precisely defined as:[OH − ]total = [OH − ]base + [OH − ]waterNormally, [OH − ]water is 1 × 10 −7 M and is muchless than [OH − ]base, thus we can usually ignore it.BUT, in this example, [OH − ]base turns out to beonly 1 × 10 −14 M, much less than the [OH − ]water.So in this example we can actually ignore the tinyamount of OH − contributed from the base and onlyconsider the OH − naturally present in water:This will work for base concentrations of 1 × 10 −8 M andlower. It gets complicated mathematically right around1 × 10 −7 M (not dealt with in this course).
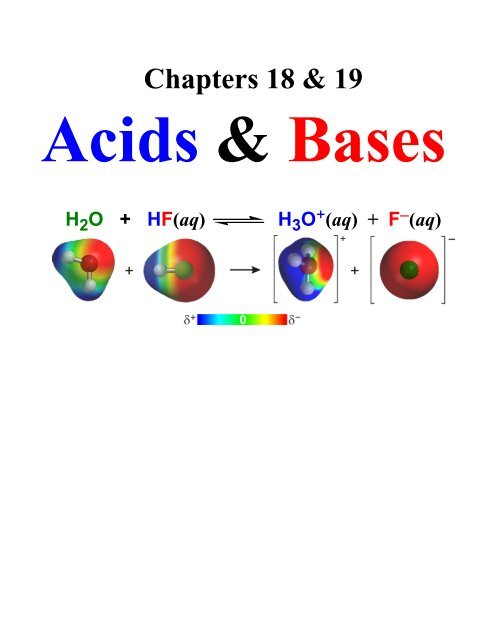
Conjugate Acid-Base Pairs<strong>Acids</strong> & <strong>Bases</strong> 23Bronsted-Lowry Definition of a Base: a substancethat combines or accepts a H + . Consider thedissociation of an acid:H 2 O + HF(aq) H 3 O + (aq) + F − (aq)Because this is an equilibrium, F − (aq) is back reacting withH + (aq) to produce undissociated acid HF(aq). Therefore,F − (aq) is acting like a base! Since it was originally part of theacid (HF), there’s a special name for it: conjugate base.Strong acids have weak conjugate bases.Weak acids have stronger conjugate bases(but usually not as strong as OH − ).To indicate that an equilibrium favors one side of arxn chemists sometimes use the double equilibriumarrows where one arrow is shorter. For a strongacid (weak conjugate base) one could write:HCl(aq) H + (aq) + Cl − (aq)
<strong>Acids</strong> & <strong>Bases</strong> 24Bronsted-Lowry Definition of a Acid: a substancethat donates a H + .Consider the reaction of a weak base with water:NH 3 (aq) + H 2 ONH 4 + (aq) + OH − (aq)Because this is an equilibrium, NH 4+ (aq) donates a H + (aq)that reacts with OH − (aq) to produce the original NH 3 (andwater). Therefore, NH 4+ (aq) is acting like an acid! Since itstarted as a weak base (NH 3 ), we call NH 4+ a conjugate acid.Strong bases have weak conjugate acids.Weak bases have stronger conjugate acids.In the two reactions shown on this page and the previous,water is acting as either an acid or a base. Any chemical thatcan act as either an acid or base is called amphoteric.In the following reaction, Na + is more accurately calleda conjugate Lewis Acid (not a proton donor).NaOH(aq)Na + (aq) + OH − (aq)
<strong>Acids</strong> & <strong>Bases</strong> 25Weak <strong>Acids</strong>These are acids that only dissociate to produce asmall amount of H + in solution:HF(aq)Ka+−H+ (aq) + F − (aq)[ H ][ F ]= = 3.5×10 −4[ HF]Most of a weak acid is dissolved in solution in itsundissociated, neutral form. An acid is weakbecause its conjugate base (counter-anion) is agood base and likes to bind to H + .A weak acid typically has K a < 1 × 10 −3Most weak acids are organic acids based on thecarboxylic group:Some of the more common weak acids that we runinto on a daily basis include:
<strong>Acids</strong> & <strong>Bases</strong> 26Acetic Acid Carbonic Acid Phosphoric AcidOOOH 3 CCOHHOCOHHOPOH OHactive ingredientin vinegarformed when CO 2dissolves in waterused in soda(Coke, Pepsi)Acetylsalicylic AcidOAscorbic AcidH 3 CHCOCCOCOHHOHOCH2HHOOHCCCHHOOHHAspirinVitamin CNote that the “OH” groups in all these examples are nothydroxides!! The oxygen atom of the OH is strongly bondedto the atom they are attached to and will not fall off as OH − .Instead they dissociate H + due to the O’s electronegativityand ability to stabilize negative charge(s).
<strong>Acids</strong> & <strong>Bases</strong> 27Example: What is the pH of a 0.1 M solution ofacetic acid? K a ≅ 1 × 10 −5Abbreviation for acetic acid: HOAcInitial Cond: 0.1 M 0 M 0 MHOAc(aq)H + (aq) + OAc − (aq)@ Equilib: 0.1 - x x xSubstitute our x values into the equilibriumexpression and solve for x:[+ −H ][ OAc ]− 5Ka= = 1×10[ HOAc]( x)( x)(0.1 − x)= 1×10− 5But, this will be a quadratic expression and we'llhave to use the quadratic formula to solve for x(ugh!).There is, however, a very good approximation wecan make to dramatically simplify the algebra.
<strong>Acids</strong> & <strong>Bases</strong> 28Because K a is fairly small (10 −5 ) we know thatacetic acid is a weak acid and that we are onlygoing to make small quantities of H + . That meansthat x should be a rather small number as well.This in turn means that 0.1 − x should be ≈ 0.1.Our assumption, therefore, will be that x will bemuch less than 0.1 M (initial concentration ofacid). This really simplifies the algebra:( x)( x)= 1×10−5(0.1 − x)assume that x
When can I drop x??When is it a good approximation??<strong>Acids</strong> & <strong>Bases</strong> 29When K eq is 1 × 10 −3 or smaller and x works out tobe at least an order of magnitude smaller than theinitial concentration that one is subtracting x from.This also works when one is adding x to the initialconcentration (e.g., common ion problems).Problem: What is the pH of a 10 M solution ofhydrogen sulfide? K a ≅ 1 × 10 −7H 2 S(aq)H + (aq) + HS − (aq)
<strong>Acids</strong> & <strong>Bases</strong> 30Problem: Prof. Stanley makes a new organic acid.He prepares a 0.01 M solution and finds that thepH is 4.0. What is the K a for this new acid?HA(aq)H + (aq) + A − (aq)Problem: What is the pH of a 0.01 M solution ofHCN? K a ≅ 4 × 10 −10
Effect of Structure on Acid/Base Behavior<strong>Acids</strong> & <strong>Bases</strong> 31Hydrohalic <strong>Acids</strong>A combination of factors affects the acid strengthof hydrohalic acids:1) Polarity of the H-X bondElectrostatic2) Strength of the H-X bond attraction!!3) Stability of the conjugate base, X −Thus, HF is a weak acid because the rather smallfluoride ion (F − ) has a concentrated negativecharge that very effectively and strongly attractsthe H + cation, not allowing it to dissociate andbecome a strong acid (like HCl, HBr, or HI).HF HCl HBr HI
<strong>Acids</strong> & <strong>Bases</strong> 32OxyacidsOxyacids are those acids in which the central atom(most commonly N, Cl, S or P) is bonded to at leastone, and usually more, oxygen atoms. Theresulting negative charge on this unit is balancedby the proper # of H + that associate with theoxygen atoms (one per oxygen atom).A list of common oxyacids and their names:HNO 2 HNO 3NitrousNitricHClO HClO 2 HClO 3 HClO 4Hypochlorous Chlorous Chloric PercloricH 2 SO 3 H 2 SO 4SulfurousSulfuricH 3 PO 2 H 3 PO 3 H 3 PO 4Hypophosphorous Phosphorus Phosphoric
<strong>Acids</strong> & <strong>Bases</strong> 33The strength of oxyacids increases for a series ofcompounds as follows:1) given the same central atom, the more oxygenspresent the stronger the acidReasoning: the more electronegative oxygen atomspresent, the more the negative charge on the anion isspread out over a larger volume. This means that thenegative charge will be less concentrated on any singleoxygen. Thus, there will be a lower electrostatic attractionto the H + cations, allowing them to dissociate more easily.2) for the same # of oxygens, the more electronegativethe central atom, the stronger the acidReasoning: the more electronegative the central atom, themore the negative charge on the anion will be pulledtowards the central atom and away from the outlyingoxygens. Thus, there will be a lower electrostaticattraction to H + cations, allowing easier dissociation.3) For almost all acids, the higher the negativecharge on the anion (conjugate base), the lowerthe acidity of the acid.Reasoning: the higher the negative charge on the anion(mono- or polyatomic) the stronger the electrostaticattraction to the H + cations. This makes it harder for theH + cation to dissociate.
<strong>Acids</strong> & <strong>Bases</strong> 344) For almost all acids, the more electronegativeatoms present (like O or F) the higher theacidity of that acid.Reasoning: the more electronegative atoms present, themore the negative charge on the anion will be pulledtowards these atoms and away from the atom that the H + isassociated with.Example:HClO HClO 2 HClO 3 HClO 4Hypochlorous Chlorous Chloric PercloricK a = 3 x 10 −8 K a = 1 x 10 −2 K a = 5 x 10 2 K a ≈ 1 x 10 10Electrostatic charge potential (ECP) surface plots for ClO −through ClO 4−anions (no H + ). The red color (dark) indicatesmore negative charge and a stronger electrostatic attractionto the H + cation (weaker acid). Positive charge is indicatedby the blue color (darker color on center atom).
Here are the ECP surface plots for the acids:<strong>Acids</strong> & <strong>Bases</strong> 35HClO HClO 2 HClO 3 HClO 4Note that the blue area around the H atom indicating positivecharge is increasing as O atoms are added.Problem: Which is the stronger acid of the pair?a) H 2 SO 4 or H 3 PO 4b) HNO 2 or HNO 3c) HOBr or HOId) H 2 SeO 4 or H 2 SeO 3e) HI or HFf) H 2 SO 3 or H 2 SO 4g)OOHHCCOHorFFCCOHHF
Weak <strong>Bases</strong><strong>Acids</strong> & <strong>Bases</strong> 36These are bases that only react to a relatively smallextent with H + in solution::NH 3 (aq) + H + (aq) NH + 4 (aq)The convention, however, for writing equilibria forweak bases is to react the base with water, whichgenerates a small quantity of OH-::NH 3 (aq) + H 2 O NH + 4 (aq) + OH − (aq)This is the equilibrium we use to define our baseequilibrium constant, K b :[ NH+ −4 ][ OH ]− 5Kb= = 1.8×10[ NH]3In this equilibrium NH + 4 (aq) is called theconjugate acid (it acts like a weak acid).
<strong>Acids</strong> & <strong>Bases</strong> 37Some weak bases are shown in the table below:Base Formula Structure K bmethylamine NH 2 CH 3 N CHH 34.4 x 10-4carbonate ion CO 32-OHO 2-1.8 x 10-4COammonia NH 3 H N H 1.8 x 10-5hydrosulfide ion HS-H S 1.8 x 10-7Hnicotine C 10 H 14 N 2N7 x 10-7NCH 3 1.4 x 10-11hydroxylamine NH 2 OH H N OH 1.1 x 10-8HpyridineC 5 H 5 NN1.9 x 10-9Note that all bases have atoms with lone pairs ofelectrons that can interact with a H + . Rememberthat a H + doesn't have any electrons and has apositive charge. It will be attracted to atoms withnegative charges and/or lone pairs of electrons.
<strong>Acids</strong> & <strong>Bases</strong> 38Example: What is the pH of a 0.1 M solution ofammonia? K b ≅ 1 × 10 −5Init: 0.1 M 0 M 0 M:NH 3 (aq) + H 2 ONH 4 + (aq) + OH − (aq)@ Eq: 0.1 - x x xSubstitute our x values into the equilibriumexpression and solve for x:[ NH+ −4 ][ OH ]− 5Kb= = 1×10[N H ]( x)( x)(0.1 − x)3= 1×10− 5But, this will be a quadratic expression and thequadratic formula is needed to exactly solve for x(ugh!).But lets use the very good approximation fromweak acid equilibria problem solving that willdramatically simplify the algebra.
<strong>Acids</strong> & <strong>Bases</strong> 39Because K b is pretty small (10 −5 ) we know thatammonia is a weak base and that we are only goingto make small quantities of OH − . That means thatx will be a pretty small number. This in turnmeans that 0.1 - x will be ∼ 0.1.Our assumption, therefore, will be that x will bemuch less than 0.1 M (initial concentration of acid).This now really simplifies our algebra:( x)( x)(0.1 − x)= 1×10( x)( x)= 1×10(0.1)x 2 = 1×10−6}− 5− 5assume that x
<strong>Acids</strong> & <strong>Bases</strong> 40Problem: What is the pH of a 1 M solution ofsodium carbonate? K b ≅ 1 × 10 −4CO 3 2− (aq) + H 2 OHCO 3 − (aq) + OH − (aq)Problem: What is the pH of a 0.01 M solution ofNH 2 OH? K b ≅ 1 × 10 −8
Lewis <strong>Acids</strong> & <strong>Bases</strong><strong>Acids</strong> & <strong>Bases</strong> 41Acid electron acceptorBase electron donorAlthough the Lewis definition includes the“traditional” Arrhenius and Brønsted-Lowry acids& bases mentioned so far, it also encompassesmolecules that don’t. Foremost are metal atomsthat form bonds to other molecules using emptyorbitals on the metal (Lewis acid) and filled lonepairs on the donor atoms (Lewis bases).Transition metal atoms typically form thestrongest bonds, followed by actinide andlanthanides. Some examples are shown below:ClPR 3OsHPhOOWCl H H 3 C NPR 3O H 2ClProblem: Identify the Lewis Base and Acid parts ofeach compound shown above. Extra: What is theoxidation state of the metal in each compound?H 2NR 3 PClPdPR 3
<strong>Acids</strong> & <strong>Bases</strong> 42Another class of important Lewis acids aretrivalent compounds of boron and aluminum thathave an empty orbital present. These are typicallyvery reactive to donor molecules including water.BF 3 , for example, is considered a “superacid.”FClBAlF F Cl ClBCl 3 will form a moderately strong bond to :NH 3 :ClHHHClHB + NB NClClHClClHProblem: What is the hybridization of the B in BCl 3 ?What about the B in Cl 3 B:NH 3 ?
<strong>Acids</strong> & <strong>Bases</strong> 43Relationship Between K a and K bConsider our ammonia equilibrium::NH 3 (aq) + H 2 O NH + 4 (aq) + OH − (aq)K+ −[ NH4][ OH ]b = [ NH3]−[ OH ] =Kwbut remember that :[ H+]substituting this in for [ O H−]we now have :Kb=+4[ NH ] Kw[ NH] [H+]3This expression now corresponds to the followingequilibrium multiplied times K w ::NH 3 (aq) + H + (aq) NH + 4 (aq)The reverse of this reaction, however, is the "acid"equilibrium:NH + 4 (aq) :NH 3 (aq) + H + (aq)Writing the reaction this way means that we cannow set-up a K a equilibrium expression:
Ka=[ NH3][H+[ NH ]4+]<strong>Acids</strong> & <strong>Bases</strong> 44But note that the reciprocal of this expression isalready in the Kb relationship:Ka=[ NH3][H+[ NH ]4+] NH+4Kb=[ ] K[ NH ] [ HTherefore, K a and K b are related via K w .Here are the very important K a / K b relationshipsyou need to know/understand:KwK b = -or- Ka=Ka3w+]KKwbK = K × Kwab
<strong>Acids</strong> & <strong>Bases</strong> 45Knowing the mathematical relationship betweenK a and K b is very important. Many referencesonly list K a values for bases and not the K b valuesthat you would expect.When a K a value is given for a base it is really thevalue for the conjugate acid of that base. Forexample:Base = NH 3 K b = 1.8 × 10 −5Conjugate Acid = [NH 4 ] + K a = 5.6 × 10 −10(K b )(K a ) = (1.8 × 10 −5 )(5.6 × 10 −10 ) = 1 × 10 −14K wIf a reference gives a K a value for the base you arelooking up, you'll have to convert it to a K b valuein order to calculate the [OH − ], and then the pH.You must, therefore, pay close attention to whatdata the problem is giving you and what you needto use. READ CAREFULLY!!DANGER!!VERY Common mistake!!
<strong>Acids</strong> & <strong>Bases</strong> 46Problem: What is the pH of a 0.1 M solution of theweak base triethyl amine (NEt 3 )? K a = 1 × 10 −11Problem: What is the pH of a 0.01 M solution ofhydroxyamine (NH 2 OH)? K a = 1 × 10 −6
Salts of Weak <strong>Acids</strong> and <strong>Bases</strong><strong>Acids</strong> & <strong>Bases</strong> 47When a weak acid (e.g., acetic acid) reacts with astrong base (e.g., an alkali hydroxide like NaOH)water and the salt of the weak acid is formed:Note that the acetate anion formed in the reactionis itself a weak base. Thus it can react with waterto produce a small amount of [OH − ].Therefore, dissolving sodium acetate (the salt ofacetic acid) in water will make a basic solution.
<strong>Acids</strong> & <strong>Bases</strong> 48Similarly, the reaction of a weak base with a strongacid will produce the salt of a weak base, which willact as a weak acid. Shown below we have thereaction of ammonia with HCl to produceammonium chloride (the salt of a weak base):Dissolving ammonium chloride in water, therefore,produces an acidic solution (NH 4+ is a weak acid).
<strong>Acids</strong> & <strong>Bases</strong> 49The big question is “How the heck do I tell whethera salt will produce an acidic, basic, or neutralsolution??”The KEY is to remember the strong acids andbases:Strong <strong>Acids</strong>:HCl(aq)H + (aq) + Cl − (aq)HBr(aq)H + (aq) + Br − (aq)HI(aq)H + (aq) + I − (aq)HNO 3(aq)H + (aq) + NO − 3(aq)H 2SO 4(aq) 2H + (aq) + SO − 4(aq) *HClO 4(aq)H + (aq) + ClO − 4(aq)Strong <strong>Bases</strong>:LiOH(aq)NaOH(aq)KOH(aq)RbOH(aq)CsOH(aq)Li + (aq) + OH − (aq)Na + (aq) + OH − (aq)K + (aq) + OH − (aq)Rb + (aq) + OH − (aq)Cs + (aq) + OH − (aq)These areextremelyweakconjugatebases --NeutralAnions!These areextremelyweakconjugateacids --NeutralCations!Putting these Neutral (neutral here refers to theiracid-base properties, NOT their charges!!) anionsand cations together generates Neutral (not acidicor basic) salts (Ca 2+ , Sr 2+ , Ba 2+ salts not shown):
NeutralCationNeutralAnionNeutral SaltsLi + Cl − LiCl, LiBr, LiI, LiNO 3 , Li 2SO 4,<strong>Acids</strong> & <strong>Bases</strong> 50Na + Br − NaCl, NaBr, NaI, NaNO 3 , Na 2 SO 4 ,K + I − KCl, KBr, KI, KNO 3 , K 2 SO 4 ,Rb + NO 3−RbCl, RbBr, RbI, RbNO 3 , Rb 2 SO 4 ,Cs + SO 42−CsCl, CsBr, CsI, CsNO 3 , Cs 2 SO 4 ,ClO 4−Perchlorate salts are explosive !!!Solutions of these Neutral Salts are neither acidicnor basic, but rather have a pH = 7 (Neutral!).A simple set of guidelines, therefore, are:CATIONS other than Li + , Na + , K + , Rb + , Cs + (&Ca 2+ , Sr 2+ , Ba 2+ ) will generate ACIDIC solutions(that is, the cation is a good conjugate acid)ANIONS other than Cl − , Br − , I − , NO 3 − , SO 42− willgenerate a BASIC solution (that is, the anion is agood conjugate base)
<strong>Acids</strong> & <strong>Bases</strong> 51SALTSCation(+)Anion(-)Cation comes from the base Anion comes from the acidIdentify the CationIs the cation from a strong base ( Group 1A or Ca2+, Sr2+, Ba2+)YESIdentify the anionIs it from a ?strong acidNOCation is from a weak base(cation is a conjugate acid)YESNOSalt Solutionwill beNeutral(Salt of astrongacid & base)Anion is froma weak acid(anion is amoderate togood conjugatebase)Identify the anionIs the anion from a strong acid?YESSalt solutionwill be acidic(salt of aweak base)NOSalt solutionwill be basic(salt of aweak acid)Salt of aweak acid &a weak base!Complicated!Don’t worry
<strong>Acids</strong> & <strong>Bases</strong> 52Problem: Will the following salts make an acidic,basic or neutral solution when dissolved in water?a) NaF K a (HF) = 3.5 × 10 -4b) NaCl K a (HCl) = 1 × 10 8c)N H + Cl - K a (pyridineH + ) = 5 × 10 -6d) C 6 H 5 COONa K a (C 6 H 5 COOH) = 6.5 × 10 -5e) RbBr K a (HBr) = 1 × 10 10f) CH 3 COOCs K a (CH 3 COOH) = 1.7 × 10 -5g) Cs 2 S K a (H 2 S) = 9.1 × 10 -8h) NH 4 NO 3 K a (NH + 4 ) = 5.6 × 10 -10i) KCN K a (HCN) = 4.9 × 10 -10j) KNO 3
Example: What is the pH of 1 M NaF?K a (HF) = 1 × 10 −4 .NeutralCationInitial: 1 M 0 0F − (aq) + H 2 O<strong>Acids</strong> & <strong>Bases</strong> 53BasicAnionHF(aq) + OH − (aq)@ Eq: 1 - x x xThis is a basic equilibrium, so we need to convertK a into K b :Kb−14Kw1×10= = = 1×10− 4K 1×10a−10Our equilibrium expression, therefore, is:Kb[ HF][ OH ]= = 1×10[ F−]( x)( x)(1 − x)−−10assume that x
So the [OH − ] = 1 × 10 −5 M. The pOH is:pOH = -log(1 × 10 −5 ) = 5The pH, then, is given by:pH = 14 - pOH = 14 - 5 = 9<strong>Acids</strong> & <strong>Bases</strong> 54DANGER!!VERYCommonmistake!!
<strong>Acids</strong> & <strong>Bases</strong> 55Problem: What is the pH of a 0.1 M solution ofNH 4 Cl. K a (NH 4 + ) = 1 x 10 −9 .
Reactions of <strong>Acids</strong> & <strong>Bases</strong><strong>Acids</strong> & <strong>Bases</strong> 56Strong <strong>Acids</strong> and Strong <strong>Bases</strong>:The reaction of a strong acid and strong base is thesimplest type of acid-base reaction.Consider the rxn of NaOH and HCl:Na + (aq) + OH − (aq) + H + (aq) + Cl − (aq)H 2 O + Na + (aq) + Cl − (aq)The rxn above shows all the aqueous species. Note thatNaCl(aq) is one of the products. When acids react withbases, water and the salt of the acid/base are formed (thebase provides the cation and acid the anion).The net ionic equation is shown below:OH − (aq) + H + (aq) H 2 OA key point to remember is that when we reactacids and bases we are usually reacting solutions ofacids and bases. When one mixes two solutionstogether the overall volume increases and theconcentrations of all species will decrease!Because of the change in volume due to the mixingtogether of two solutions one has to take this intoaccount. DANGER!! VERY Common mistake!!We do this by dealing directly with MOLES andnot Molarity. So you have to:
• convert molarity into moles,• do your calculations, then<strong>Acids</strong> & <strong>Bases</strong> 57• convert back to molarity using the NEW total(combined) solution volumeExample: What is the pH if we mix 100 mL of 0.1M HCl with 50 mL of 0.1 M NaOH?First, convert concentrations into moles:(100 mL)(0.1 M) = 10 mmol HCl(50 mL)(0.1 M) = 5 mmol NaOHNow for the reaction: 5 mmol NaOH will reactwith only 5 mmol HCl, leaving behind 5 mmol HCl.But now we have 150 mL of solution, so [H + ] is:DANGER!! VERY Common mistake!!5 mmol150 mL = 0.033 M H+ } p H = 1.5
<strong>Acids</strong> & <strong>Bases</strong> 58Problem: What will be the pH when 100 mL of0.1 M HNO 3 is mixed with 300 mL of 0.2 M KOH?Problem: What will be the pH when 100 mL of0.1 M HNO 3 is mixed with 200 mL of 0.05 MKOH?
<strong>Acids</strong> & <strong>Bases</strong> 59Strong <strong>Acids</strong> and Weak <strong>Bases</strong>:Strong <strong>Bases</strong> and Weak <strong>Acids</strong>:Things get more complicated (well, not too bad)when you react a strong acid with a weak base or astrong base with a weak acid.For this case, however, we will simplify things alittle by only using equivalent amounts of the acidand base.The key thing to remember when you react a weakacid or base with a strong base or acid is that oneends up with the SALT of that weak acid or base.Remember that the SALT of a weak acid is actuallya weak base and gives a basic solution.Similarly, the SALT of a weak base generates anacidic solution.DANGER!!VERY Common mistake!!
<strong>Acids</strong> & <strong>Bases</strong> 60Example: What is the pH if 150 mL of 0.5 Macetic acid is mixed with 150 mL of 0.5 M NaOH?Ka (HOAc) ≅ 1 × 10 −5 .HOAc(aq) + OH − (aq) H 2 O + OAc − (aq)The OAc − produced is a weak base and willproduce a weakly basic solution:H 2 O + OAc − (aq) HOAc(aq) + OH − (aq)Since this is a base equilibrium, we need to use K b :KKbb−14Kw1×10= = = 1×10− 5K 1×10a[ HOAc][OH ]= = 1×10[ OAc−]−− 9Not realizing this - DANGER!!VERY Common mistake!!− 9Calculate the concentration of OAc − :(150 mL)(0.5 M) = 75 mmol HOAc(150 mL)(0.5 M) = 75 mmol OH −Not realizing this - DANGER!!VERY Common mistake!!
<strong>Acids</strong> & <strong>Bases</strong> 61Thus, we will produce 75 mmol of OAc − . Theconcentration of OAc − , therefore, will be:75 mmol= 0.25M OAc −300 mLNow we can set-up our initial and @eq conditions:Volume Change -DANGER!!VERY Commonmistake!!Initial: 0.25 M 0 0H 2 O + OAc − (aq)HOAc(aq) + OH − (aq)@ Eq: 0.25 - x x xKb[ HOAc][ OH ]= = 1×10[ OAc−]( x)( x)(0.25 − x)= 1×10( x)( x)= 1×10(0.25)x 2 = 2.5×10−x = 1.6×105−10−9=−− 9}[OH−]−9assume that x
<strong>Acids</strong> & <strong>Bases</strong> 62Problem: What is the pH of the reaction of500 mL of 2 M NH 3 and 500 mL of 2 M HCl?Ka (NH 4 + ) ≅ 1 × 10 −10 .
<strong>Acids</strong> & <strong>Bases</strong> 63TitrationsA titration is the careful measured addition of aknown concentration of one substance that willreact with another unknown material in order todetermine the concentration of the unknownmaterial.Determining the concentrations of unknown materials is aroutine procedure in chemistry. Titrations are commonlyused to determine the concentrations of acids and bases insolution, as well as many other chemicals. Titrations are alsooften the simplest and least expensive way of determiningconcentrations of unknown materials.If we are titrating an unknown acid with a knownamount of base with a known concentration, wecan use the (M 1 )(V 1 ) = (M 2 )(V 2 ) relationship tofind the unknown's concentration at theequivalence point (the point at which we've addedjust enough base to react with all the acid).In order to determine when we have reached theequivalence point in an acid base reaction, wegenerally use an indicator.
<strong>Acids</strong> & <strong>Bases</strong> 64IndicatorsAn indicator is a weak organic acid or base thathas sharply different colors in its associated anddissociated forms:HIn(aq)H + (aq) + In − (aq)red acidicblue basicIndicators usually have very intense colors so oneonly has to use a very small amount (a few drops)so it will not affect the titration of the solution.Remember that the indicator is an acid or base so ifyou add a lot it will affect the titration!!OHPhenophtaleinOOHOO+ H +HOacidic form - colorlessHOanionic basic form - redOOHMethyl RedOONNN N NN + H +acidic form - red anionic basic form - yellow
Some Common Acid-Base Indicators<strong>Acids</strong> & <strong>Bases</strong> 65NamepH color changeregion Acid color Base colorMethyl violet 0 - 2 yellow violetmethyl yellow 1.2 - 2.3 red yellowmethyl orange 2.9 - 4.0 red yellowmethyl red 4.2 - 6.3 red yellowbromthymol blue 6.0 - 7.6 yellow bluethymol blue 8.0 - 9.6 yellow bluephenolphthalein 8.3 - 10 colorless pinkAlizarin yellow 10.1 - 12 yellow redHIn(aq)Ka=H + (aq) + In − (aq)+−[ H ][ In][ HI n]At the color change [H + ] = [In − ] = [HIn], so:
<strong>Acids</strong> & <strong>Bases</strong> 66Titrating an unknown strong acid with a knownamount of strong base:
<strong>Acids</strong> & <strong>Bases</strong> 67Titrating an unknown strong base with a knownamount of strong acid:
<strong>Acids</strong> & <strong>Bases</strong> 68Titrating an unknown weak base with a knownamount of strong acid:
<strong>Acids</strong> & <strong>Bases</strong> 69Titrating an unknown weak acid with a knownamount of strong base:
<strong>Acids</strong> & <strong>Bases</strong> 70Buffer SolutionsConsider an acetic acid solution:HOAc(aq)H + (aq) + OAc − (aq)If we add enough NaOAc (the salt of acetic acid) toincrease the OAc − concentration roughly equal toHOAc, we now form the following mixture:HOAc(aq)H + (aq) + OAc− (aq)The added OAc − , which is a weak base, will consumesome of the free H + causing the pH to rise (become lessacidic).Note that if we add H + to this solution, it will react withthe large pool of weak base OAc − to form HOAc. TheH + concentration, therefore, will stay about the same.Similarly, if we add some OH − it will react with the H +present. Since we have an equilibrium, however, someHOAc will, in turn, dissociate to replace the missing H + ,keeping it about the same.A solution of a weak acid and the salt (conjugate base)of a weak acid – or – a weak base and the salt(conjugate acid) of a weak base is called a BUFFER!
<strong>Acids</strong> & <strong>Bases</strong> 71If the concentration of the two components is highenough and you don’t add too much acid or base, thebuffer solution will be very resistant to changes in the pHdue to added acid or base.Buffer solutions are critically important to biologicalsystems (i.e., keeping us alive!).For a solution of a weak acid or base and their salt, onecan write the following equations for calculating the[H + ] and [OH − ] of the buffer solution generated:[ Base][ OH−⎛⎞] = K b ⎜ ⎟⎝ [ acidic salt ] ⎠⎛ [ Acid]⎞[ H+] = Ka⎜[ basic salt]⎟⎝⎠These are called the Henderson-Hasselbalchequations.The log form of these equations:⎛[ basic salt]⎞pH= pKa+ log⎜ ⎟⎝ [ acid]⎠pOH⎛[ acidic salt]⎞= pKb+ log⎜ ⎟⎝ [ base]⎠
<strong>Acids</strong> & <strong>Bases</strong> 72If the acid/base concentration is the same as thesalt concentration, then we can write:[OH − ] = K b–– or ––[H + ] = K apOH = pK b pH = pK aBy varying the weak acid or base and the salt beingused to make the buffer solution, as well as theirconcentration ratio, one can set the pH of thebuffer to almost anything you want.The more concentrated the buffer components, themore effective the buffer solution will be atresisting pH changes. But remember that you canalways overload a buffer by adding too much acidor base to it.One of the trickiest things for you to determine isjust what salts will work to make a buffer. Thesalts of strong acids and bases (i.e., NaCl, KBr,Na 2 SO 4 , CsNO 3 ) do NOT usually make buffers,nor do mixtures of strong acids and bases withtheir salts!
<strong>Acids</strong> & <strong>Bases</strong> 73Note, however, that reacting ½ equivalent of astrong base with a weak acid, generates a buffersolution!This is the region where wehave a buffer solution present
<strong>Acids</strong> & <strong>Bases</strong> 74Problem: Which of the following solutions is atraditional buffer prepared from a weak acid/baseand a conjugate salt?a) 0.1 M NaOH + 0.1 M NaOAcb) 0.01 M HCl + 0.01 M NaClc) 0.5 M NH 4 Cl + 0.5 M NH 3d) 2 M NaOAc + 2 M HOAce) 0.01 M citric acid + 0.01 M sodium citratef) 0.3 M H 3 PO 4 + 0.3 M NaH 2 PO 4g) 2 M HNO 3 + 2 M NaNO 3h) 0.05 M H 2 CO 3 + 0.05 M KHCO 3i) 0.5 M HI + 0.5 M CsIj) 0.2 M benzoic acid + 0.2 M cesium benzoatek) 1 M NaOAc + 1 M KBrl) 0.001 M HCl + 0.001 M KOHm) 0.1 M KOAc + 0.1 M NH 3n) 0.2 M HOAc + 0.2 M KHCO 3b) Which will be the most effective buffer solutionat maintaining a given pH?c) What are the pH’s of the buffer solutions?