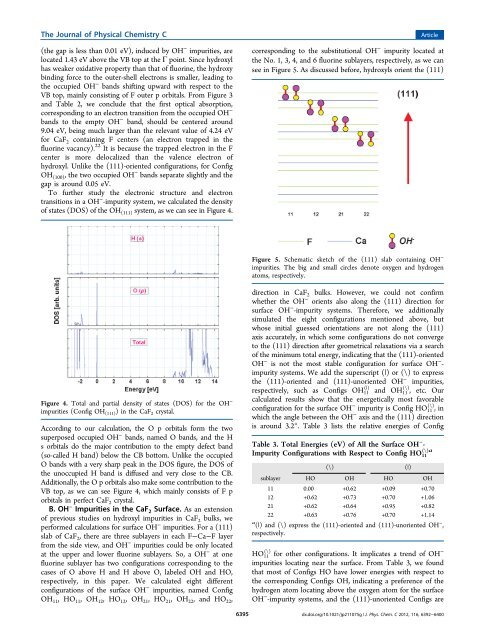
The Journal <strong>of</strong> Physical Chemistry C(the gap is less than 0.01 eV), <strong>in</strong>duced by OH − impurities, arelocated 1.43 eV above the VB top at the Γ po<strong>in</strong>t. S<strong>in</strong>ce hydroxylhas weaker oxidative property than that <strong>of</strong> fluor<strong>in</strong>e, the hydroxyb<strong>in</strong>d<strong>in</strong>g force to the outer-shell electrons is smaller, lead<strong>in</strong>g tothe occupied OH − bands shift<strong>in</strong>g upward with respect to theVB top, ma<strong>in</strong>ly consist<strong>in</strong>g <strong>of</strong> F outer p orbitals. From Figure 3and Table 2, we conclude that the first optical absorption,correspond<strong>in</strong>g to an electron transition from the occupied OH −bands to the empty OH − band, should be centered around9.04 eV, be<strong>in</strong>g much larger than the relevant value <strong>of</strong> 4.24 eVfor CaF 2 conta<strong>in</strong><strong>in</strong>g F centers (an electron trapped <strong>in</strong> thefluor<strong>in</strong>e vacancy). 22 It is because the trapped electron <strong>in</strong> the Fcenter is more delocalized than the valence electron <strong>of</strong>hydroxyl. Unlike the (111)-oriented configurations, for ConfigOH (100) , the two occupied OH − bands separate slightly and thegap is around 0.05 eV.To further study the electronic structure and electrontransitions <strong>in</strong> a OH − -impurity system, we calculated the density<strong>of</strong> states (DOS) <strong>of</strong> the OH (111) system, as we can see <strong>in</strong> Figure 4.Articlecorrespond<strong>in</strong>g to the substitutional OH − impurity located atthe No. 1, 3, 4, and 6 fluor<strong>in</strong>e sublayers, respectively, as we cansee <strong>in</strong> Figure 5. As discussed before, hydroxyls orient the (111)Figure 5. Schematic sketch <strong>of</strong> the (111) slab conta<strong>in</strong><strong>in</strong>g OH −impurities. The big and small circles denote oxygen and hydrogenatoms, respectively.Figure 4. Total and partial density <strong>of</strong> states (DOS) for the OH −impurities (Config OH (111) ) <strong>in</strong> the CaF 2 crystal.Accord<strong>in</strong>g to our calculation, the O p orbitals form the twosuperposed occupied OH − bands, named O bands, and the Hs orbitals do the major contribution to the empty defect band(so-called H band) below the CB bottom. Unlike the occupiedO bands with a very sharp peak <strong>in</strong> the DOS figure, the DOS <strong>of</strong>the unoccupied H band is diffused and very close to the CB.Additionally, the O p orbitals also make some contribution to theVB top, as we can see Figure 4, which ma<strong>in</strong>ly consists <strong>of</strong> F porbitals <strong>in</strong> perfect CaF 2 crystal.B. OH − <strong>Impurities</strong> <strong>in</strong> the CaF 2 Surface. As an extension<strong>of</strong> previous studies on hydroxyl impurities <strong>in</strong> CaF 2 bulks, weperformed calculations for surface OH − impurities. For a (111)slab <strong>of</strong> CaF 2 , there are three sublayers <strong>in</strong> each F−Ca−F layerfrom the side view, and OH − impurities could be only locatedat the upper and lower fluor<strong>in</strong>e sublayers. So, a OH − at onefluor<strong>in</strong>e sublayer has two configurations correspond<strong>in</strong>g to thecases <strong>of</strong> O above H and H above O, labeled OH and HO,respectively, <strong>in</strong> this paper. We calculated eight differentconfigurations <strong>of</strong> the surface OH − impurities, named ConfigOH 11 , HO 11 , OH 12 , HO 12 , OH 21 , HO 21 , OH 22 , and HO 22 ,6395direction <strong>in</strong> CaF 2 bulks. However, we could not confirmwhether the OH − orients also along the (111) direction forsurface OH − -impurity systems. Therefore, we additionallysimulated the eight configurations mentioned above, butwhose <strong>in</strong>itial guessed orientations are not along the (111)axis accurately, <strong>in</strong> which some configurations do not convergeto the (111) direction after geometrical relaxations via a search<strong>of</strong> the m<strong>in</strong>imum total energy, <strong>in</strong>dicat<strong>in</strong>g that the (111)-orientedOH − is not the most stable configuration for surface OH − -impurity systems. We add the superscript (|) or (\) to expressthe (111)-oriented and (111)-unoriented OH − impurities,(|)respectively, such as Configs OH 11 and OH (\) 11 , etc. Ourcalculated results show that the energetically most favorableconfiguration for the surface OH − impurity is Config HO (\) 11 ,<strong>in</strong>which the angle between the OH − axis and the (111) directionis around 3.2°. Table 3 lists the relative energies <strong>of</strong> ConfigTable 3. Total Energies (eV) <strong>of</strong> All the Surface OH − -(\)aImpurity Configurations with Respect to Config HO 11(\) (|)sublayer HO OH HO OH11 0.00 +0.62 +0.09 +0.7012 +0.62 +0.73 +0.70 +1.0621 +0.62 +0.64 +0.95 +0.8222 +0.63 +0.76 +0.70 +1.14a (|) and (\) express the (111)-oriented and (111)-unoriented OH − ,respectively.HO (\) 11 for other configurations. It implicates a trend <strong>of</strong> OH −impurities locat<strong>in</strong>g near the surface. From Table 3, we foundthat most <strong>of</strong> Configs HO have lower energies with respect tothe correspond<strong>in</strong>g Configs OH, <strong>in</strong>dicat<strong>in</strong>g a preference <strong>of</strong> thehydrogen atom locat<strong>in</strong>g above the oxygen atom for the surfaceOH − -impurity systems, and the (111)-unoriented Configs aredx.doi.org/10.1021/jp211075g | J. Phys. Chem. C 2012, 116, 6392−6400
The Journal <strong>of</strong> Physical Chemistry CArticlethe more energetically favorable configurations with respect tothe correspond<strong>in</strong>g (111)-oriented Configs. Additionally, wealso calculated the (111) CaF 2 slabs full covered by OH − . Forthe full-covered slabs, hydroxyls can only replace the surfacefluor<strong>in</strong>e sublayer, so we may classify the full-covered slabs <strong>in</strong>t<strong>of</strong>our configurations, i.e., HO (|) full ,HO (\) full ,OH (|) full , and OH (\) full . Ourcalculated results demonstrate that the most stable full-coveredconfiguration is Config HO (\) full , <strong>in</strong> which the deviation angleequals around 2.2°, and the total energies <strong>of</strong> Configs HO (|) full ,OH (|) full , and OH (\) full are larger than that <strong>of</strong> Config HO (\) full by 0.06,0.63, and 0.58 eV, respectively. So, we can conclude that for thefull-covered slabs, hydrogen atoms prefer to locate aboveoxygen atoms obliquely. Configs HO (\) 11 and HO (\) full are morestable than other surface OH − systems; therefore, we ma<strong>in</strong>lyfocus our current discussion on these two Configs.The geometrical structures <strong>of</strong> the surface OH − are calculatedand depicted <strong>in</strong> Table 4. Accord<strong>in</strong>g to our studies on bulkTable 4. Geometrical Properties <strong>of</strong> the Surface OH −<strong>Impurities</strong> a for all Configs(\) (|)sublayer HO OH HO OH11 0.96(3.2°) 0.97(28°) 0.97 0.9812 0.97(5.3°) 0.97(58.1°) 0.97 0.9621 0.97(65.0°) 0.96(4.8°) 0.96 0.9722 0.97(5.0°) 0.96(57.9°) 0.97 0.95full 0.96(2.2°) 0.97(2.5°) 0.97 0.98a OH − length (Å) and deviation angle shown <strong>in</strong> brackets.OH − -impurity systems, the lengths <strong>of</strong> OH − <strong>in</strong> CaF 2 and BaF 2 ,as well as <strong>in</strong> H 2 O, Ca(OH) 2 , and Ba(OH) 2 , are all around0.96−0.97 Å, <strong>in</strong>dicat<strong>in</strong>g that the OH − as a diatomic group has asteady geometrical structure. Our calculated CaF 2 surface OH − -impurity lengths for all the configurations are around 0.96−0.98 Å,as we can see <strong>in</strong> Table 4. It is implied that the surface effect onthe length <strong>of</strong> surface hydroxyls is not remarkable. From Table4, we found that some Configs, such as OH (\) 12 ,HO (\) 21 , andOH (\) 22 , have very big deviation angles (around 60°), and theorientations <strong>of</strong> other Configs approach the (111) direction. Inan ideal cubic CaF 2 bulk, the angle between the (111) and(100) axes is 54.7°, be<strong>in</strong>g close to those big obliquities <strong>of</strong>around 60°. Therefore, we can consider these Configs with bigdeviation angles as Config OH (100) , namely, the (100)-orientedconfiguration <strong>in</strong> the bulk case, locat<strong>in</strong>g near the (111) surface.It is implied that despite the (111)-oriented OH − be<strong>in</strong>genergetically more favorable <strong>in</strong> CaF 2 bulk, hydroxyls located atsome specific fluor<strong>in</strong>e sublayers near the surface prefer the(100) orientation. Here, we can classify the eight (111)-unorientedconfigurations <strong>in</strong>to three OH − types, i.e., OH (111) ,HO (111) ,andOH (100) <strong>in</strong> the bulk case. Configs OH (\) 11 ,HO (\) 12 ,OH (\) (\)21 ,andHO 22belong to the OH (111) type, Config HO (\) 11 belongs to the HO (111)type, and Configs OH (\) 12 , HO (\) (\)21 , and OH 22 belongs to theOH (100) type. From Tables 3 and 4 and this classification, we canconclude that OH − impurities with HO (111) ,OH (111) ,OH (100) ,andOH (111) configurations prefer to locate at the No.1, 3, 4, and 6fluor<strong>in</strong>e sublayers, respectively.The relaxations <strong>of</strong> atoms surround<strong>in</strong>g the surface OH −impurity are shown <strong>in</strong> Figure 6 and Table 5. For ConfigHO (\) 11 , all the atoms near the surface shift toward the positiveY-axis from the top view, as we can see Figure 6. The oxygenand the six nearest fluor<strong>in</strong>e atoms, i.e., F1, F2, and F3, on theupper sublayer <strong>of</strong> the first layer have considerable XY-shifts6396Figure 6. Top view <strong>of</strong> the surface OH − -impurity nearest-neighborgeometry with an <strong>in</strong>dication <strong>of</strong> relaxation shifts for Config HO (\) 11 . Thedirections <strong>of</strong> atomic displacements <strong>in</strong> the XY-plane are shown witharrows. The fluor<strong>in</strong>e and calcium atoms <strong>in</strong> different shells are labeled.The upper and lower panels denote the first and second layers <strong>of</strong> the(111) CaF 2 slab, respectively.over 2% <strong>of</strong> a 0 , the XY-displacements <strong>of</strong> fluor<strong>in</strong>e atoms locatedat the lower sublayer <strong>of</strong> the first layer and the upper sublayer <strong>of</strong>the second layer are around 1−2% <strong>of</strong> a 0 , and the XYtranslations<strong>of</strong> the fluor<strong>in</strong>e atoms on the lower sublayer <strong>of</strong> thesecond layer equal 0.7% <strong>of</strong> a 0 approximately. Here, we canconclude that the atomic layers conta<strong>in</strong><strong>in</strong>g surface OH −impurities have a remarkable XY-translation. Because <strong>of</strong> thesurface shr<strong>in</strong>k<strong>in</strong>g effect, the atomic coord<strong>in</strong>ates <strong>of</strong> most <strong>of</strong> thesurface atoms reduce, whereas the oxygen atom shifts outwardfrom the surface by around 2.22% <strong>of</strong> a 0 . Table 5 also lists thedisplacements <strong>of</strong> atoms at the top two layers for Config HO (\) full ,i.e., the full-covered case. Similar to Config HO (\) 11 , the toplayers have an obvious XY-translation with respect to thedeeper layers and the surface OH − layer moves upward byaround 3.32% <strong>of</strong> a 0 , implicat<strong>in</strong>g a dilat<strong>in</strong>g effect <strong>in</strong>duced by fullcover<strong>in</strong>ghydroxyls.Table 6 presents the effective charges <strong>of</strong> the surface OH − forall the (111)-unoriented configurations. The OH − charges formost Configs are larger than the OH − charge <strong>in</strong> the CaF 2 bulk(−0.722 e). Especially for Config HO (\) 11 , the effective charge <strong>of</strong>the surface OH − equals −0.746 e and is larger by around 0.024 e.Because <strong>of</strong> the surface effect, −0.005 e localized on thehydrogen atom transfers <strong>in</strong>ward toward the surface and the oxygenatom attracts −0.028 e from the surround<strong>in</strong>g atoms. We alsocalculated the effective charges <strong>of</strong> atoms surround<strong>in</strong>g the OH −impurity,aswecansee<strong>in</strong>Table5.Thechargedifferences<strong>of</strong>thedeeper fluor<strong>in</strong>e and calcium atoms are negligible, whereas thedx.doi.org/10.1021/jp211075g | J. Phys. Chem. C 2012, 116, 6392−6400