mdg-annual-report-2013
mdg-annual-report-2013
mdg-annual-report-2013
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
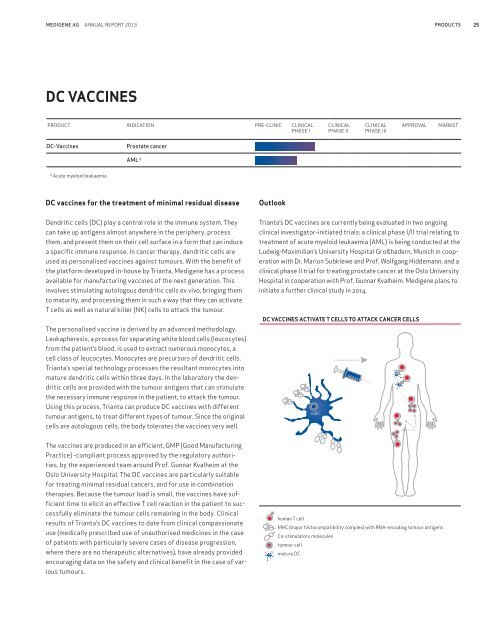
Medigene AG Annual <strong>report</strong> <strong>2013</strong> products 25DC vaccinesProduct Indication Pre-clinic ClinicalPhase IClinicalPhase IIClinicalPhase IIIApprovalMarketDC-VaccinesProstate cancerAML 1)1)Acute myeloid leukaemiaDC vaccines for the treatment of minimal residual diseaseOutlookDendritic cells (DC) play a central role in the immune system. Theycan take up antigens almost anywhere in the periphery, processthem, and present them on their cell surface in a form that can inducea specific immune response. In cancer therapy, dendritic cells areused as personalised vaccines against tumours. With the benefit ofthe platform developed in-house by Trianta, Medigene has a processavailable for manufacturing vaccines of the next generation. Thisinvolves stimulating autologous dendritic cells ex vivo, bringing themto maturity, and processing them in such a way that they can activateT cells as well as natural killer (NK) cells to attack the tumour.The personalised vaccine is derived by an advanced methodology.leukapheresis, a process for separating white blood cells (leucocytes)from the patient’s blood, is used to extract numerous monocytes, acell class of leucocytes. Monocytes are precursors of dendritic cells.Trianta’s special technology processes the resultant monocytes intomature dendritic cells within three days. In the laboratory the dendriticcells are provided with the tumour antigens that can stimulatethe necessary immune response in the patient, to attack the tumour.Using this process, Trianta can produce DC vaccines with differenttumour antigens, to treat different types of tumour. Since the originalcells are autologous cells, the body tolerates the vaccines very well.Trianta’s DC vaccines are currently being evaluated in two ongoingclinical investigator-initiated trials: a clinical phase I/II trial relating totreatment of acute myeloid leukaemia (AML) is being conducted at theLudwig-Maximilian’s University Hospital Großhadern, Munich in cooperationwith Dr. Marion Subklewe and Prof. Wolfgang Hiddemann, and aclinical phase II trial for treating prostate cancer at the Oslo UniversityHospital in cooperation with Prof. Gunnar Kvalheim. Medigene plans toinitiate a further clinical study in 2014.DC vaccines activate T cells to attack cancer cellsmDCThe vaccines are produced in an efficient, GMP (Good ManufacturingPractice) -compliant process approved by the regulatory authorities,by the experienced team around Prof. Gunnar Kvalheim at theOslo University Hospital. The DC vaccines are particularly suitablefor treating minimal residual cancers, and for use in combinationtherapies. Because the tumour load is small, the vaccines have sufficienttime to elicit an effective T cell reaction in the patient to successfullyeliminate the tumour cells remaining in the body. Clinicalresults of Trianta’s DC vaccines to date from clinical compassionateuse (medically prescribed use of unauthorised medicines in the caseof patients with particularly severe cases of disease progression,where there are no therapeutic alternatives), have already providedencouraging data on the safety and clinical benefit in the case of varioustumours.human T cellMHC (major histocompatibility complex) with RNA-encoding tumour antigensCo-stimulatory moleculestumour cellmature DC