mdg-annual-report-2013
mdg-annual-report-2013
mdg-annual-report-2013
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
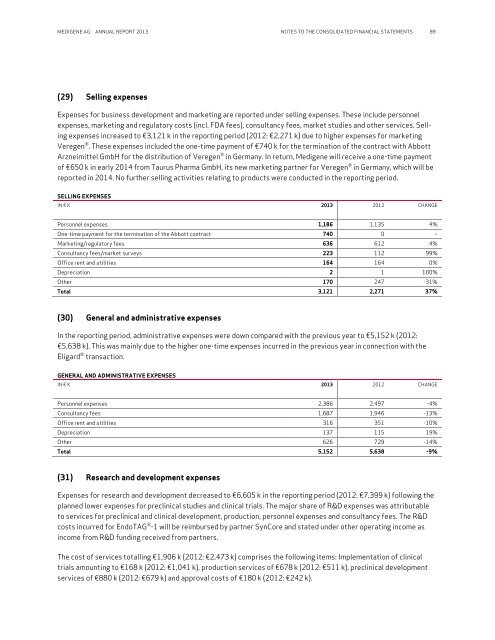
MEDIGENE AG ANNUAL REPORT <strong>2013</strong> NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS 89(29) Selling expensesExpenses for business development and marketing are <strong>report</strong>ed under selling expenses. These include personnelexpenses, marketing and regulatory costs (incl. FDA fees), consultancy fees, market studies and other services. Sellingexpenses increased to €3,121 k in the <strong>report</strong>ing period (2012: €2,271 k) due to higher expenses for marketingVeregen ® . These expenses included the one-time payment of €740 k for the termination of the contract with AbbottArzneimittel GmbH for the distribution of Veregen ® in Germany. In return, Medigene will receive a one-time paymentof €650 k in early 2014 from Taurus Pharma GmbH, its new marketing partner for Veregen ® in Germany, which will be<strong>report</strong>ed in 2014. No further selling activities relating to products were conducted in the <strong>report</strong>ing period.SELLING EXPENSESIN € K <strong>2013</strong> 2012 CHANGEPersonnel expenses 1,186 1,135 4%One-time payment for the termination of the Abbott contract 740 0 ‒Marketing/regulatory fees 636 612 4%Consultancy fees/market surveys 223 112 99%Office rent and utilities 164 164 0%Depreciation 2 1 100%Other 170 247 -31%Total 3,121 2,271 37%(30) General and administrative expensesIn the <strong>report</strong>ing period, administrative expenses were down compared with the previous year to €5,152 k (2012:€5,638 k). This was mainly due to the higher one-time expenses incurred in the previous year in connection with theEligard ® transaction.GENERAL AND ADMINISTRATIVE EXPENSESIN € K <strong>2013</strong> 2012 CHANGEPersonnel expenses 2,386 2,497 -4%Consultancy fees 1,687 1,946 -13%Office rent and utilities 316 351 -10%Depreciation 137 115 19%Other 626 729 -14%Total 5,152 5,638 -9%(31) Research and development expensesExpenses for research and development decreased to €6,605 k in the <strong>report</strong>ing period (2012: €7,399 k) following theplanned lower expenses for preclinical studies and clinical trials. The major share of R&D expenses was attributableto services for preclinical and clinical development, production, personnel expenses and consultancy fees. The R&Dcosts incurred for EndoTAG ® -1 will be reimbursed by partner SynCore and stated under other operating income asincome from R&D funding received from partners.The cost of services totalling €1,906 k (2012: €2,473 k) comprises the following items: Implementation of clinicaltrials amounting to €168 k (2012: €1,041 k), production services of €678 k (2012: €511 k), preclinical developmentservices of €880 k (2012: €679 k) and approval costs of €180 k (2012: €242 k).