Chapter 6 The polyatomic molecules
Chapter 6 The polyatomic molecules
Chapter 6 The polyatomic molecules
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
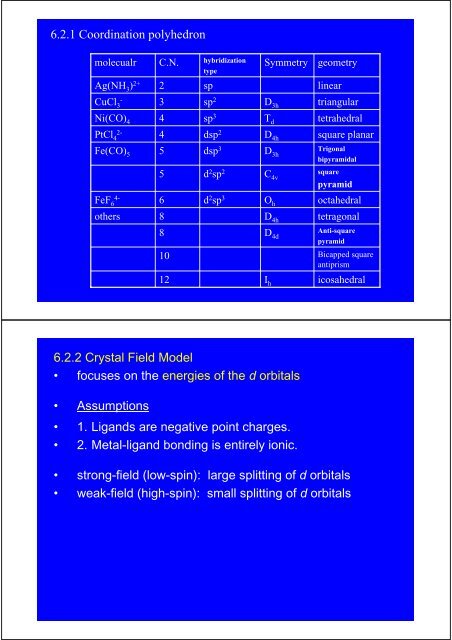
6.2.1 Coordination polyhedronmolecualrAg(NH 3 ) 2+C.N.2hybridizationtypespSymmetrygeometrylinearCuCl 3-3sp 2D 3htriangularNi(CO) 44sp 3T dtetrahedralPtCl 42-4dsp 2D 4hsquare planarFe(CO) 555dsp 3d 2 sp 2D 3hC 4vTrigonalbipyramidalsquarepyramidFeF 64-6d 2 sp 3O hoctahedralothers8D 4htetragonal810D 4dAnti-squarepyramidBicapped squareantiprism12I hicosahedral6.2.2 Crystal Field Model• focuses on the energies of the d orbitals.• Assumptions• 1. Ligands are negative point charges.• 2. Metal-ligand bonding is entirely ionic.• strong-field (low-spin): large splitting of d orbitals• weak-field (high-spin): small splitting of d orbitals