Chapter 6 The polyatomic molecules
Chapter 6 The polyatomic molecules
Chapter 6 The polyatomic molecules
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
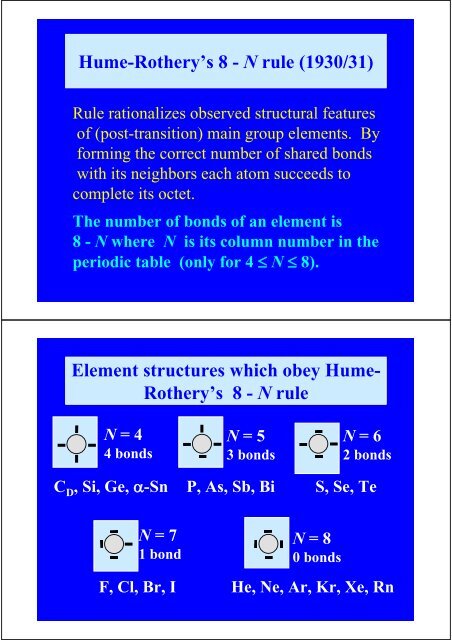
%O T and %O [3] and %O [4] = 0Hume-Rothery’s 8 - N rule (1930/31)Rule rationalizes observed structural featuresof (post-transition) main group elements. Byforming the correct number of shared bondswith its neighbors each atom succeeds tocomplete its octet.<strong>The</strong> number of bonds of an element is8 - N where N is its column number in theperiodic table (only for 4 ≤ N ≤ 8).Element structures which obey Hume-Rothery’s 8 - N ruleN = 44 bondsN = 53 bondsN = 62 bondsC D , Si, Ge, α-Sn P, As, Sb, Bi S, Se, TeN = 71 bondN = 80 bondsF, Cl, Br, I He, Ne, Ar, Kr, Xe, Rn