Chapter 6 The polyatomic molecules
Chapter 6 The polyatomic molecules
Chapter 6 The polyatomic molecules
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
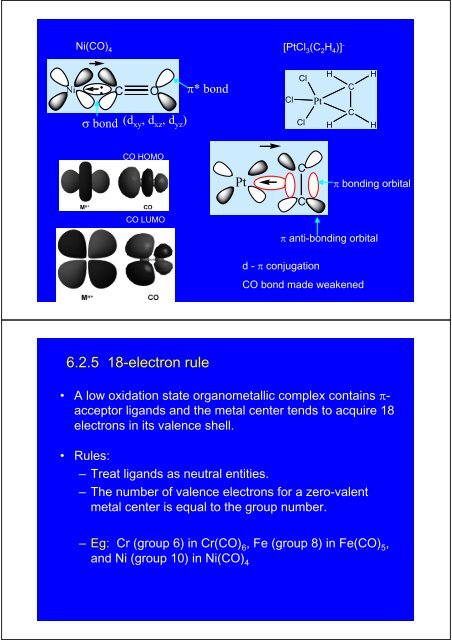
Ni(CO) 4[PtCl 3 (C 2 H 4 )] -Ni C O π* bondσ bond (d xy , d xz , d yz )ClClClPtHHCCHHCO HOMOPtCπ bonding orbitalCCO LUMOπ anti-bonding orbitald - π conjugationCO bond made weakened6.2.5 18-electron rule• A low oxidation state organometallic complex contains π-acceptor ligands and the metal center tends to acquire 18electrons in its valence shell.• Rules:– Treat ligands as neutral entities.– <strong>The</strong> number of valence electrons for a zero-valentmetal center is equal to the group number.–Eg: Cr (group 6) in Cr(CO) 6 , Fe (group 8) in Fe(CO) 5 ,and Ni (group 10) in Ni(CO) 4