REDOX & Electrochemistry - LSU Chemistry
REDOX & Electrochemistry - LSU Chemistry
REDOX & Electrochemistry - LSU Chemistry
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
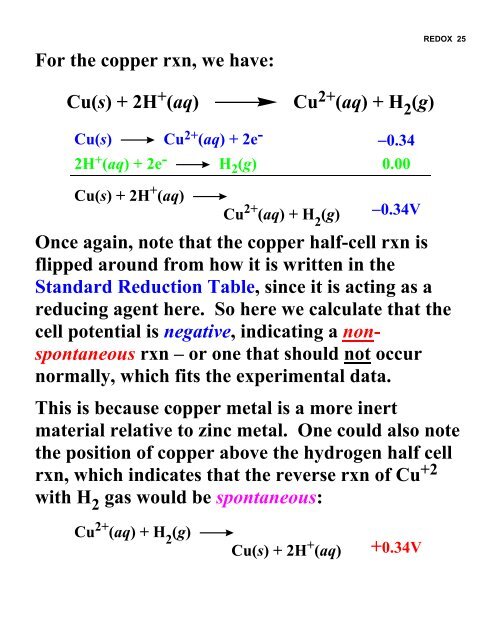
For the copper rxn, we have:<strong>REDOX</strong> 25Cu(s) + 2H + (aq)Cu 2+ (aq) + H 2 (g)Cu(s) Cu 2+ (aq) + 2e - −0.342H + (aq) + 2e - H 2 (g) 0.00Cu(s) + 2H + (aq)Cu 2+ (aq) + H 2(g)−0.34VOnce again, note that the copper half-cell rxn isflipped around from how it is written in theStandard Reduction Table, since it is acting as areducing agent here. So here we calculate that thecell potential is negative, indicating a nonspontaneousrxn – or one that should not occurnormally, which fits the experimental data.This is because copper metal is a more inertmaterial relative to zinc metal. One could also notethe position of copper above the hydrogen half cellrxn, which indicates that the reverse rxn of Cu +2with H 2 gas would be spontaneous:Cu 2+ (aq) + H 2(g)Cu(s) + 2H + (aq)+0.34V