REDOX & Electrochemistry - LSU Chemistry
REDOX & Electrochemistry - LSU Chemistry
REDOX & Electrochemistry - LSU Chemistry
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
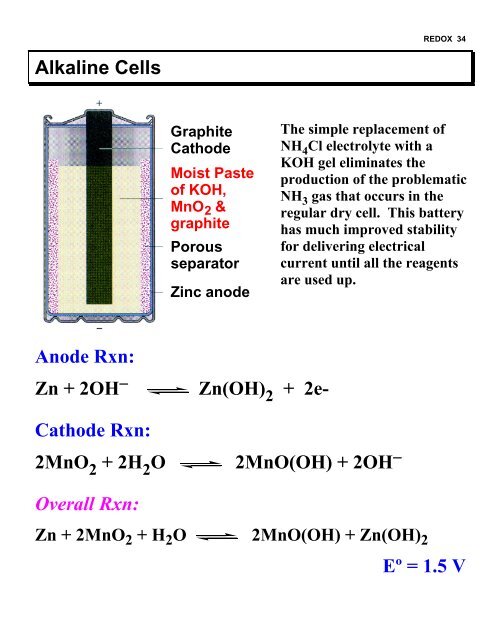
<strong>REDOX</strong> 34Alkaline CellsGraphiteCathodeMoist Pasteof KOH,MnO 2 &graphitePorousseparatorZinc anodeThe simple replacement ofNH 4 Cl electrolyte with aKOH gel eliminates theproduction of the problematicNH 3 gas that occurs in theregular dry cell. This batteryhas much improved stabilityfor delivering electricalcurrent until all the reagentsare used up.Anode Rxn:Zn + 2OH − Zn(OH) 2 + 2e-Cathode Rxn:2MnO 2 + 2H 2 O2MnO(OH) + 2OH −Overall Rxn:Zn + 2MnO 2 + H 2 O 2MnO(OH) + Zn(OH) 2Eº = 1.5 V