Lecture Notes (PDF) - Aqueous and Environmental Geochemistry
Lecture Notes (PDF) - Aqueous and Environmental Geochemistry
Lecture Notes (PDF) - Aqueous and Environmental Geochemistry
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
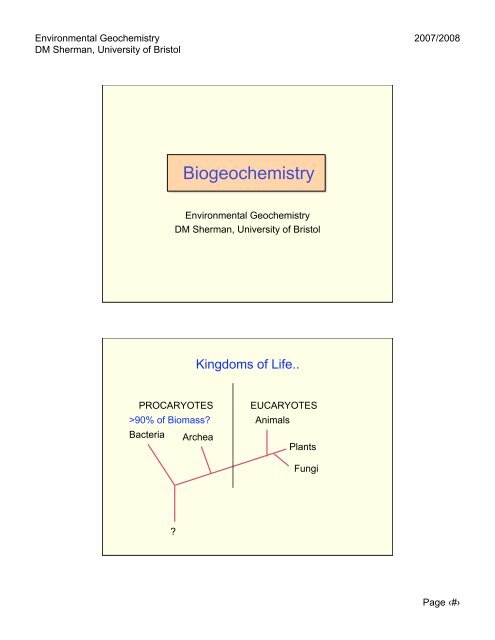
<strong>Environmental</strong> <strong>Geochemistry</strong>DM Sherman, University of Bristol2007/2008Biogeochemistry<strong>Environmental</strong> <strong>Geochemistry</strong>DM Sherman, University of BristolKingdoms of Life..PROCARYOTES>90% of Biomass?Bacteria ArcheaEUCARYOTESAnimalsPlantsFungi?Page ‹#›
<strong>Environmental</strong> <strong>Geochemistry</strong>DM Sherman, University of Bristol2007/2008Metabolic Processes•Heterotrophs: obtain carbon <strong>and</strong> energy from existingorganic carbon.•Autotrophs: reduce CO 2 to obtain organic C. Twokinds of autotrophs:•Photosynthetic organisms: obtain energy fromsunlight.•Chemolithoautotrophs: obtain energy by oxidizinginorganic compounds (Fe 2+ , NH 4+ , H 2 ).Heterotrophic Metabolism•Respiration: oxidation of organic carbon to CO 2 .This requires an electron acceptor such as O 2 :CH 2 O + O 2 = CO 2 + H 2 O•Fermentation: in the absence of an electronacceptor, organic C will play both roles:3CH 2 O + H 2 O = CO 2 + 2CH 3 OHPage ‹#›
<strong>Environmental</strong> <strong>Geochemistry</strong>DM Sherman, University of Bristol2007/2008Redox Couples Used By OrganismsBiological process arecoupled to geochemistry byredox reactions. Lifeexploits redox disequilbrium.DesulfovibrioHeterotrophic respiration with differentelectron acceptorsMetabolic ReactionCH 2O + O 2→ CO 2+ H 2OΔG (kJ/mole CH 2 O)-4935CH 2O + 4NO-3→-4724HCO-3+ CO 2+ 2N 2+ 3H 2OCH 2O + 3CO 2+ 2MnO 2+ H 2O →-3484HCO-3+ 2Mn 2+CH 2O + 7CO 2+ 4Fe 3+ + H 2O →8HCO 3-+ 4Fe 2+ + 3H 2 O-1032CH 2O + SO2-4→ 2HCO-3+ H 2S-992CH 2O → CO 2+ CH 4-88Page ‹#›
<strong>Environmental</strong> <strong>Geochemistry</strong>DM Sherman, University of Bristol2007/2008Anoxic Conditions in Estuarine SedimentsAs organic matter isconsumed by respiration, O 2is depleted <strong>and</strong> new electronacceptors are used.Anoxic mudHall Lake, WA (Balistrieri et al., 1994)Page ‹#›
<strong>Environmental</strong> <strong>Geochemistry</strong>DM Sherman, University of Bristol2007/2008MethanogenesisMethanogens are archea whichlive under extremely anaerobicconditions.CO 2 reduction pathway:4H 2 + CO 2 → CH 4 + 2H 2 OAcetate Fermentation:CH 3 COOH → CH 4 + CO 2Autotrophic Metabolism: PhotosynthesisThe overall reaction for photosynthesis isCO 2 + H 2 O → CH 2 O + O 2Page ‹#›
<strong>Environmental</strong> <strong>Geochemistry</strong>DM Sherman, University of Bristol2007/2008The Global Carbon Cycle (in 10 15 g)Atmosphere (748 as CO 2 )CO 2Dissolution/Degassing (100)Oceans(37000 as HCO 3- )0.2Rivers (0.6)Respiration (60)Photosynthesis (120)Biosphere(1600)Soil (1200-1600)Respiration (60)Volcanic Emission(0.048-0.18)Lithosphere (50 x 10 6 as CaCO 3 ; 3870 as Oil/Gas/Coal)Autotrophic OrganismsA variety of chemolithoautotrophs are found in springs <strong>and</strong>in the “deep biosphere”.Photosynthetic algeaChemolithoautotrophsPage ‹#›
<strong>Environmental</strong> <strong>Geochemistry</strong>DM Sherman, University of Bristol2007/2008Autotrophic Organisms (cont.)At black smokers at mid-oceanridge hydrothermal zones, energyis derived from sulfide oxidizingbacteria.Chemolithotrophic respiration2H 2 + O 2 → 2H 2 OMetabolic ReactionΔG (kJ/mole O 2 )-474.42H 2 S + O 2 → 2H 2 O + 2S-418.72S + O 2 + 2H 2 O → H + + HSO 44Fe 2+ + 4H + + O 2 → 2H 2 O + 4Fe 3+ -281.6NH 4++ 1.5O 2 → NO 2-+ H 2 O + 2H + -163.5Page ‹#›
<strong>Environmental</strong> <strong>Geochemistry</strong>DM Sherman, University of Bristol2007/2008Fe(II) oxidizing bacteriaGroundwater seeps from anoxic conditions have highconcentration of dissolved Fe 2+ . Chemolithoautotrophicbacteria use the Fe 2+ as a food source:4Fe 2+ + 4H + + O 2 → 2H 2 O + 4Fe 3+Iron oxidizing bacteria (Gallionella ferruginea<strong>and</strong> ) thatgrows on aquatic surfaces (Elenor Robbins, USGS)Fe(II) oxidizing bacteria (cont.)Well-fouling by Fe oxidizingbacteria.Scanning electron micrograph of a nitratedependentFe(II)-oxidizing cell highlyencrusted in iron(III) hydroxides (A. Kappler)Page ‹#›
<strong>Environmental</strong> <strong>Geochemistry</strong>DM Sherman, University of Bristol2007/2008NitrificationNitrification is the oxidation of NH 3 bychemoautotrophic organisms:NH 4++ 1.5O 2 =NO 2-+ H 2 O + 2H +(Nitrosomonas sp.)NO 2-+ 0.5 O 2 = NO 3-(Nitrobacter sp.)These reactions are critical steps in the nitrogen cycle.Biolimiting NutrientsH1Li3Na11K19Rb37Cs55Fr87Be4Mg12Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn20 21 22 23 24 25 26 27 28 29 30Sr38Ba56Ra88Y39La57Ac89P is needed for ATP synthesis.N is needed for amino acid synthesis.Metal centers are needed for 1/3 of all proteins.Zr40Hf73Nb Mo Tc41 42 43Ta73B5C6W Re Os Ir Pt Au Hg Tl Pb74 75 76 77 78 79 80 81 82U92Ru44Rh45Pd46O8F9Al Si P S Cl13 14 15 16 17Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu58 59 60 61 62 63 64 65 66 67 68 69 70 71Th90Pa91Ag47Cd48Ga31In49Ge32Sn50N7As33Sb51Bi83Se34Te52Po84Br35I53At85He2Ne10Ar18Kr36Xe54Rn86Page ‹#›
<strong>Environmental</strong> <strong>Geochemistry</strong>DM Sherman, University of Bristol2007/2008Photosynthesis <strong>and</strong> Nutrient UptakeBefore, we simplified the fixation of carbon bythe reactionCO 2 + H 2 O → CH 2 O + O 2Based on the average composition of marineplankton, a better model would be:106 CO 2 + 16 NO 3-+ HPO 4-+ 122 H 2 O + 18 H+→ C 106 H 263 O 110 N 16 P + 138 O 2C 106: N 16: P is known as the Redfield RatioNitrogen FixationNitrogen fixation is the reduction of N 2 to NH 3 for proteinsynthesis. In natural systems, nitrogen fixation is doneentirely by bacteria, archaea <strong>and</strong> cyanobacteria (blue-greenalgae).N 2 + 6e - + 6H + = 2NH 3 pK = -9.32Page ‹#›
<strong>Environmental</strong> <strong>Geochemistry</strong>DM Sherman, University of Bristol2007/2008Nitrate ReductionDenitrification is the reduction of NO 3-by heterotrophicorganisms (e.g., Pseudomonas, Micrococcus..) whichuse NO 3-as an electron acceptor under anaerobicconditions:4NO 3-+ 5“CH 2 O” +4H + = 2N 2 + 5CO 2 + 7H 2 ONitrate assimilation is the reduction of NO 3-to NH 4+byplants, fungi <strong>and</strong> bacteriaNO 3-+ 2H + + 2e- = NO 2-+ H 2 ONO 2-+ 8H + + 6e- = NH 4++ 2H 2 OThe Global Nitrogen Cycle (in 10 12 g)Atmosphere (4 x10 21 as N 2 )N 2DissolutionOceans(2.2 x10 4 N 2 ,570 NO 3- )Weathering(?)NitrificationDentrificationN assimilationN-fixationN-Fixation (300)Denitrification (300)Biosphere(3.5 x 10 4 NH 3 )OrganicBurial (40)Volcanic Emission(?)Lithosphere (1.9x10 8 ; Organics=8 x 10 8 )Page ‹#›
<strong>Environmental</strong> <strong>Geochemistry</strong>DM Sherman, University of Bristol2007/2008The Nitrogen Cycle (Terrestrial)Summary•Biology is coupled to geochemistry by exploiting redoxdisequilibria <strong>and</strong> by the uptake of nutrients.•Under anaerobic conditions, micro-organisms usealternative electron acceptors for respiration.•Chemolithoautotrophs use inorganic materials forelectron sources.•N,P <strong>and</strong> some trace metals are biolimiting nutrients.Page ‹#›