Lecture Notes (PDF) - Aqueous and Environmental Geochemistry
Lecture Notes (PDF) - Aqueous and Environmental Geochemistry
Lecture Notes (PDF) - Aqueous and Environmental Geochemistry
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
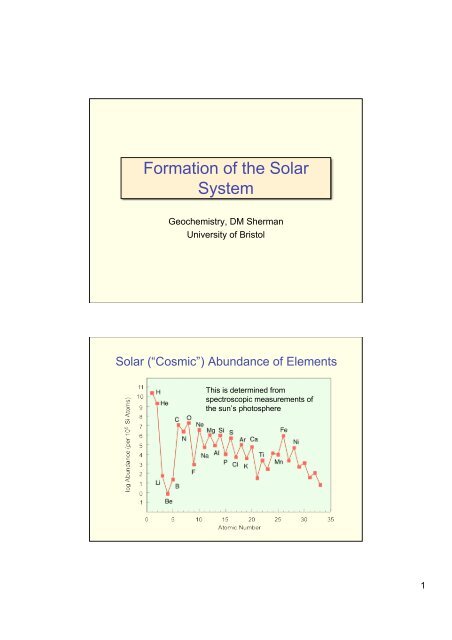
Formation of the SolarSystem<strong>Geochemistry</strong>, DM ShermanUniversity of BristolSolar (“Cosmic”) Abundance of ElementsThis is determined fromspectroscopic measurements ofthe sun’s photosphere1
The Nebular HypothesisFirst proposed in 1734 by Swedenborg.Subsequently, he was appointed by Godto reform Christianity <strong>and</strong> was able to talkto angels, demons <strong>and</strong> other spirits..Developed further by Kant in 1755. Healso developed the idea that the structureof our mind shapes all sensory experience<strong>and</strong> thought.. An idea we know take forgranted.The Nebular HypothesisMore fully developed by Laplace in 1795.Laplace was one of the greatestmathematicians of all time..A planetary systems starts out as a large(10,000 AU) cloud of interstellar dust.The cloud collapses under gravity,begins to spin <strong>and</strong> forms flat, rotatingdisk in which planets can accrete.2
Nebular HypothesisCondensation of solarnebula. Conservation ofangular momentumcreates disk.Accretion of planetesimals(T-Tauri phase of sunsweeps away volatilecomponents from inner solarsystem)Planetary differentiationSolar System Formation Animation3
Evidence forProtoplanetaryDisk formationThese are called“proplyds” byastronomers.4
Condensation of Elements…Since the nebula is nearly all hydrogen the initial partialpressure of Fe is simply0p Fe#= X g N &FeP T" %Fe( P T= 5.6 x10 )5 P T$ N H/ 2'!where P T is the total pressure (e.g., 10 -4 bar) <strong>and</strong> N Fe isthe number of Fe atoms per N H atoms.After some Fe has condensed, the partial pressure ofFe will be0p Fe= (1"#)p Fe!Condensation of Elements…We can write K as,K = (1"#)sX Fe$ N ' $&Fe) P 'T& )% N H/ 2(% (P 0!!We need the P,T dependence of K. Sinceln(K P,T) = " #G P,TRTWe getln(1"#) = " $G P,TRT "ln % N FeP ('T* + ln(X Fe&(N H/ 2)P 0 )s )!6
Condensation of Elements…Where do we get ΔG P,T ?For any pressure, temperature we have:"G P,T= "H P,T+T"S P,TIt can be shown that!!PT PT "C"G P,T= "G P0 ,T 0+ # "C pdT+TpP 0 T 0TdTPT# + # VdPP 0 T 0P 0 T 0Recall that, If ΔC p = 0 <strong>and</strong> gases are ideal, we can write:#"G P,T= "H T0 P 0+T"S P0 ,T 0+ "nRT ln P &T% ($ 'P 0!Volatile vs. Refractory Elements..Condensation of a solar gas at 10 -4 bar7
Volatile vs. Refractory Elements..Highly Refractory: Al, Ca, Ti (e.g., Al 2 O 3 )Moderately Refractory: Mg, Fe, Si (e.g., Mg 2 SiO 4 )Moderately Volatile: Na, K, S (e.g., (Na,K)AlSi 3 O 8 )Highly Volatile: H, C, N (H 2 O, CH 4 etc.)The Solar SystemGas GiantsGas GiantsTerrestrial Planets8
Solar System Differentiation by Volatility10000The “Frost Line”V E M A J S U N PTemperature (K)100010010Watercondenses at170 K10.5 1 10 50Radial Distance (AU)9
CometsVolatile (ice) rich leftovers from the outersolar nebula. Recent space probes haveshown that comets have less ice <strong>and</strong>more minerals (smectite clay, carbonates,sulfides) than expected.Meteorites as Samples of the Early SolarSystemReflectance spectrahave been used to inferthat most meteorites arefragments of asteroids.10
Meteorite Classification <strong>and</strong> OccurrenceAchondrites, Irons <strong>and</strong> Stony IronsSome meteorites are differentiated suggesting theexistence of protoplanets.Irons are thought to come fromthe cores of very small planets thatwere broken up. They consist ofFe-Ni alloy.Achondrites have no metallic ironor chondrules <strong>and</strong> are pieces of thesilicate fractions of small planets(planetismals).11
Carbonaceous ChondritesMost primitive meteoritetypes showing the leastdifferentiation.CI group is formedbelow 50 ºCCarbonaceous Chondrites (cont.)Allende meteorite•CI chondrites have evidencefor extensive aqueous alteration(serpentine, phyllosilicates) <strong>and</strong>contain up to 20% H 2 O.•Are highly oxidized withmagnetite (Fe 3 O 4 ) <strong>and</strong> sulfateminerals present.•Organic matter makes up to 30% <strong>and</strong> includespolyaromatic hydrocarbons, kerogen <strong>and</strong> amino acids.13