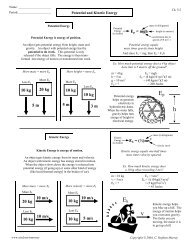
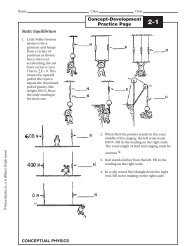
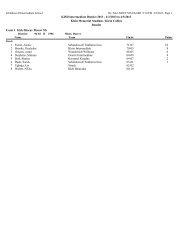
Exercise 1
Exercise 1
Exercise 1
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
StoichiometryLimiting Reactants1. If 20.0g of sodium hydroxide react with 30.0 g of sulfuric acid to producesodium sulfate, which reactant is limiting? How much sodium sulfate isproduced?2. What reactant is limiting is 3.00 g of chlorine gas reacts with 25.0 g ofsodium bromide to produce bromine gas? How much sodium chloride isproduced?3. If 20.0 g of potassium hydroxide reacts with 15.0 g of ammonium sulfate,how much ammonia is produced (the other products are potassium sulfateand water). Which reactant is limiting?4. If a drain cleaner contains 455 g of sodium hydroxide and 45.0 grams ofaluminum shavings, when this is reacted with water, how much hydrogengas would be produced (the other product is sodium aluminate (AlO 2 -1 )?What is the limiting reactant?5. If 23.4 g of sodium hydroxide reacts with 50.0 g of hydrochloric aced,how much water will be produced? What is the limiting reactant?6. How much chromium (III) hydroxide will be produced by the reaction of2.37 g of silver nitrate with 2.00 g of sodium hydroxide?7. When 84.1 g of magnesium oxide reacts with 47 g of water, how muchmagnesium hydroxide is formed? What is the limiting reactant?8. If 261 g of potassium chloride reacts with 543 g of silver nitrate, howmuch silver chloride will be produced?10-11HC/CC/TG KHS